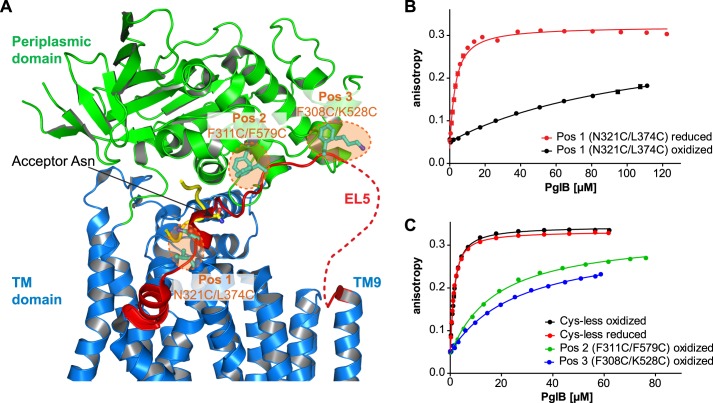
FIGURE 1.
Disulfide cross-linking of EL5 to core PglB. A, schematic representation of C. lari PglB (PDB code 3RCE) with the TM domain in blue, the periplasmic domain in green, and bound acceptor peptide in yellow. EL5 connecting TM9 and TM10 is colored red, with the structurally disordered segment (25 residues) depicted by a red dashed line. Residues selected for cross-linking are shown in a ball and stick representation and colored cyan. The pairwise mutation to cysteines at three distinct positions is illustrated by orange dashed circles, and corresponding residues are indicated. B and C, peptide binding of different cross-linking mutants quantified by fluorescence anisotropy. Purified PglB was titrated into a solution containing 1 μm fluorescently labeled peptide variant (containing the sequon DQNAT) and 10 mm MnCl2. Data points reflect the mean of 20 measurements of the same sample. Error bars, S.D. Curves labeled “oxidized” indicate samples that were cross-linked with CuCl2 during the purification process, whereas “reduced” indicates samples that contained 80 mm β-mercaptoethanol.