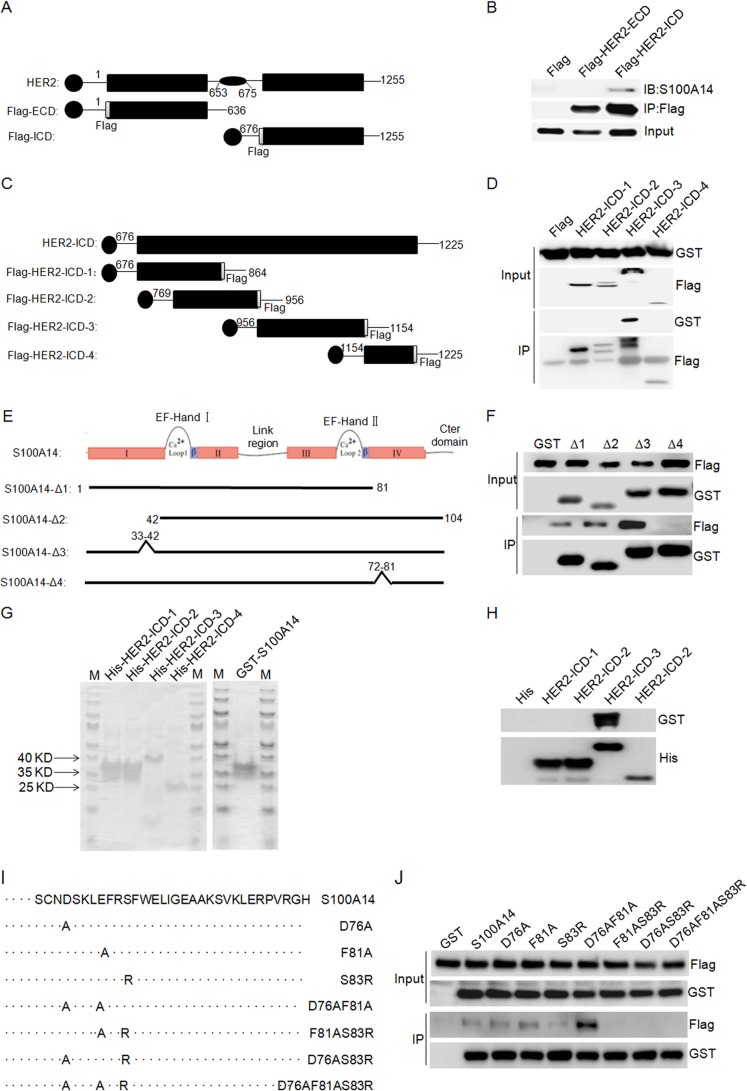
FIGURE 4.
Mapping of the S100A14 binding site in HER2 and the HER2 binding site in S100A14. A, schematic overview of HER2 ECD and ICD that were tested for S100A14 binding. The specific residues present in each fragment are indicated. HER2-ECD, HER2 extracellular domain (residues 1–636); HER2-ICD, HER2 intracellular domain (residues 676–1255). B, HER2 truncation mutants bind to immobilized S100A14. The indicated FLAG-HER2 truncations and GST-S100A14 were cotransfected into HEK 293T/17 cells as indicated, and cell lysates were then immunoprecipitated using anti-FLAG® M2 affinity gel. The immunoprecipitates were examined by Western blotting (IB) using anti-S100A14 antibody. Input represented 5% of cell lysates used in the co-IP experiment. C, schematic representation of HER2 constructs depicting the HER2 intracellular domain and HER2 intracellular domain fragments. The specific residues present in each fragment are indicated: HER2-ICD-1 (residues 676–864), HER2-ICD-2 (residues 769–956), and HER2-ICD-3 (residues 956–1154), HER2-ICD-4 (residues 1154–1255). D, HER2-ICD truncation mutants bind to immobilized S100A14. The indicated FLAG-HER2-ICD truncations and GST-S100A14 were cotransfected into HEK 293T/17 cells as indicated, and cell lysates were then immunoprecipitated using anti-FLAG® M2 affinity gel. The immunoprecipitates were examined by Western blotting using anti-S100A14 antibody. Input represented 5% of cell lysates used in the co-IP experiment. E, schematic overview of S100A14 truncation mutants that were tested for HER2 binding. S100A14-Δ1, -Δ2, -Δ3, and -Δ4, S100A14 with residues 82–104, 1–41, 33–42, and 72–81 deleted, respectively. F, S100A14 truncation mutants bind to immobilized HER2. The indicated GST-S100A14 truncations and FLAG-HER2 were cotransfected into HEK 293T/17 cells as indicated, and cell lysates were then immunoprecipitated using glutathione-Sepharose (GSH) beads. The immunoprecipitates were examined by Western blotting using anti-FLAG antibody. Input represented 5% of cell lysates used in the co-IP experiment. G, purified recombinant proteins used in the direct interaction assay. Approximately 0.2 μg of each protein was separated by SDS-PAGE and stained with Coomassie Brilliant Blue. M, molecular weight markers. H, direct interaction between GST-tagged S100A14 and His-tagged HER2 intracellular domain fragments. I, overview of the different S100A14 mutants that were tested for HER2 binding. S100A14 mutants containing point mutations in helix 4 (D76A, F81A, S83R, D76A/F81A, F81A/S83R, D76A/S83R, and D76A/F81A/S83R) are shown. J, amino acid residue Ser-83 of S100A14 is essential for HER2 binding. GST-S100A14 and corresponding mutants and FLAG-HER2 were cotransfected into HEK 293T/17 cells as indicated, and cell lysates were immunoprecipitated using glutathione-Sepharose (GSH) beads. The immunoprecipitates were examined by Western blotting using anti-FLAG antibody. Input represented 5% of cell lysates used in the co-IP experiment.