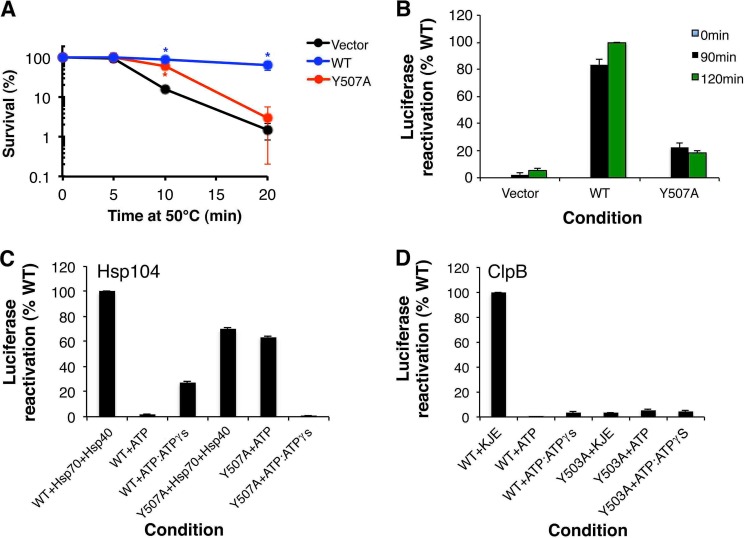
FIGURE 2.
Hsp104Y507A is a hypomorph with altered intrinsic disaggregase activity. A, Δhsp104 yeast cells harboring empty pHSE vector (black markers), pHSE-Hsp104 (blue markers), or pHSE-Hsp104Y507A (red markers) were grown to mid-log phase in SD-ura liquid. Prior to the 50 °C heat treatment, matched cultures were preincubated at 37 °C for 30 min. Following treatment at 50 °C for 0–20 min cells were transferred to ice, diluted in ice-cold SD-ura, immediately plated on SD-ura, and allowed to recover for 3 days at 30 °C and cell viability was assessed. Values represent mean ± S.E. (n = 4). A one-way ANOVA with the post hoc Dunnett's multiple comparisons test was used to compare vector alone to the Hsp104WT and Hsp104Y507A constructs (* denotes p < 0.05). B, Δhsp104 yeast cells harboring pGPD-LuxAB (encoding a temperature-sensitive luciferase fusion protein) and either empty vector (pHSE), pHSE-Hsp104, or pHSE-Hsp104Y507A were grown to mid-log phase in SD-his-ura liquid. Matched cultures were preincubated at 37 °C for 30 min and then incubated at 44 °C for 50 min. Cycloheximide (10 μg/ml) was then added and cultures were incubated for a further 10 min at 44 °C. Cells were then shifted to 30 °C and luciferase activity was measured at 0, 90, and 120 min. Luciferase activity was expressed as the % of WT Hsp104 condition after 120 min. Values represent mean ± S.E. (n = 3). C, urea-denatured firefly luciferase aggregates were incubated for 90 min at 25 °C with Hsp104 (1 μm) or Hsp104Y507A (1 μm) in the presence of ATP (5.1 mm), a mixture of ATP (1 mm) and ATPγS (4 mm), or Hsc70 (an Hsp70) (1 μm) and Hdj2 (an Hsp40) (1 μm) plus ATP (5.1 mm). Reactivation of luciferase was then determined by luminescence and converted to % WT disassembly activity (activity of 1 μm WT Hsp104 in the presence of Hsc70 and Hdj2). Values represent mean ± S.E. (n = 3). D, urea-denatured firefly luciferase aggregates were incubated for 60 min at 25 °C with ClpB (0.167 μm) or ClpBY503A (0.167 μm) in the presence of ATP (5.1 mm), a mixture of ATP (2.5 mm) and ATPγS (2.5 mm), or DnaK (1 μm), DnaJ (0.033 μm), and GrpE (0.0167 μm) plus ATP (5.1 mm). Reactivation of luciferase was then determined by luminescence and converted to % WT ClpB activity (activity of 0.167 μm WT ClpB in the presence of DnaK, DnaJ, and GrpE). Values represent mean ± S.E. (n = 3).