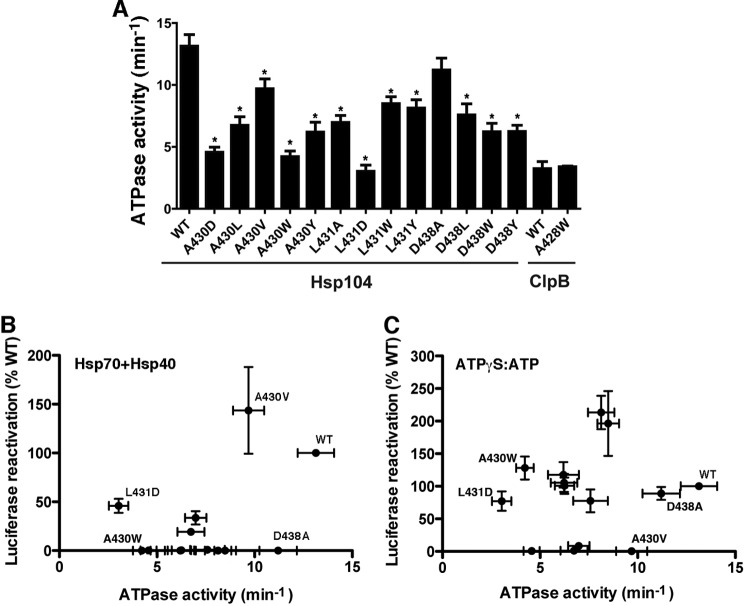
FIGURE 4.
Reduced ATPase activity does not explain the inability of specific distal loop variants to collaborate with Hsp70. A, the indicated Hsp104 or ClpB variants (0.25 μm) were incubated for 12 (Hsp104 variants) or 30 min (ClpB variants) with ATP (1 mm) and the resulting ATPase rates were determined. Values represent mean ± S.E. (n = 3–9). A one-way analysis of variance with the post hoc Dunnett's multiple comparisons test was used to compare Hsp104WT to Hsp104 variants. A t test was used to compare ClpB to ClpBA428W (* denotes p < 0.05). B, reactivation of luciferase in the presence of Hsc70, and Hdj2 is plotted against ATPase activity for WT Hsp104 and variants. Positions of WT, A430W, A430V, L431D, and D438A are indicated. Values represent mean ± S.E. (n = 3–9). A simple linear regression yielded a coefficient of determination, R2 = 0.1994. C, reactivation of luciferase in the presence of ATPγS:ATP is plotted against ATPase activity for WT Hsp104 and variants. Positions of WT, A430W, A430V, L431D, and D438A are indicated. Values represent mean ± S.E. (n = 3–10). A simple linear regression yielded a coefficient of determination, R2 = 0.012.