FIGURE 6.
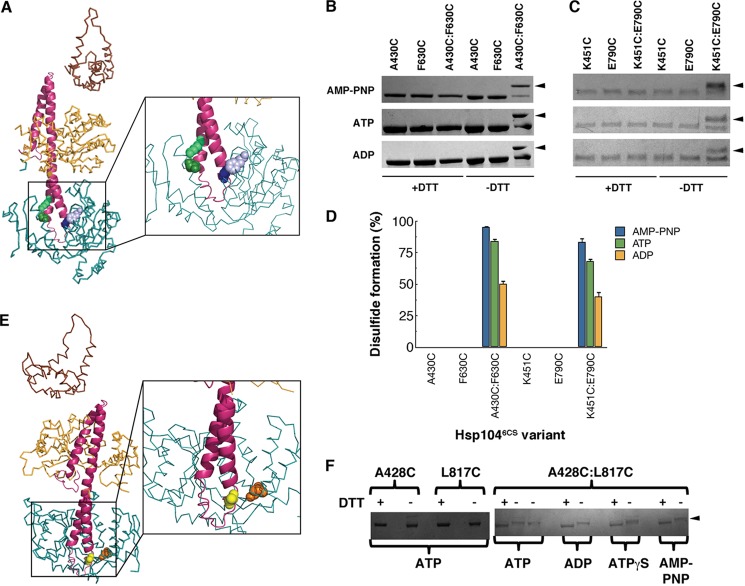
The distal loop of the MD can contact NBD2 in Hsp104 and ClpB. A, successful disulfide cross-links formed between the MD (pink) and NBD2 (blue) of Hsp104 (only one monomer shown) as predicted by the optimized Hsp104 rigid body fit of the Hsp104N728A hexamer in the presence of ATP (Fig. 5G) (52). Residue A430C (dark purple spheres) in the MD could cross-link to F630C (light purple spheres) in NBD2. Residue K451C (dark green spheres) in the MD could cross-link to E790C (light green spheres) in NBD2. B and C, intramolecular disulfide cross-links formed between A430C and F630C (B) and K451C and E790C (C) in the presence of ADP, ATP, or AMP-PNP. Reduced and oxidized Hsp104 variants (±DTT) were analyzed by non-reducing SDS-PAGE. Cross-links were visualized by band up-shift in SDS-PAGE (arrowheads indicated up-shifted species). Single cysteine mutant controls did not show cross-linking. Up-shift was seen only in double cysteine mutants. D, the extent of disulfide formation monitored as in B and C was quantified using Ellman's reagent. Values represent mean ± S.E. (n = 3). E, successful disulfide cross-links formed between the MD (pink) and NBD2 (blue) of ClpB (only one monomer shown) as deduced from the optimized Hsp104 rigid body fit of the Hsp104N728A hexamer in the presence of ATP (52). Residue A428C (yellow spheres) in the MD could cross-link to L817C (orange spheres) in NBD2. F, intramolecular disulfide cross-links formed between A428C and L817C in the presence of ADP, ATP, ATPγS, or AMP-PNP. Reduced and oxidized ClpB variants (±DTT) were analyzed by non-reducing SDS-PAGE. Cross-links were visualized by band up-shift in SDS-PAGE. Single cysteine mutant controls did not show cross-linking. Up-shift was seen only in double cysteine mutants (arrowhead indicates up-shifted species).