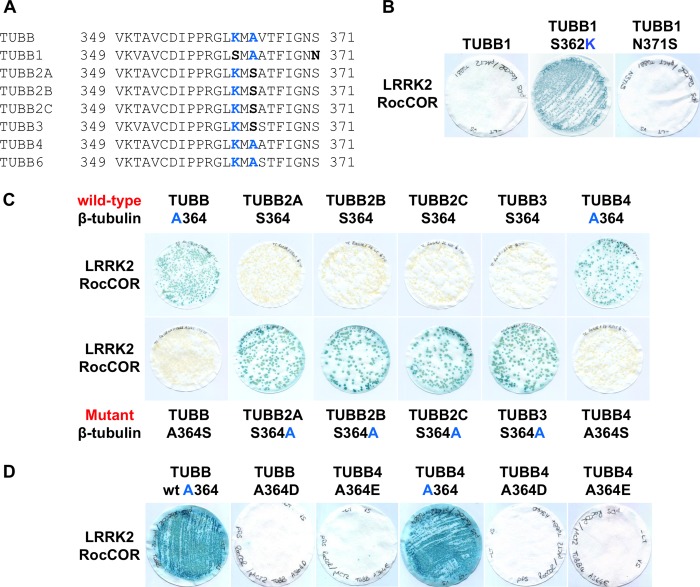
FIGURE 3.
The LRRK2 β-tubulin interaction requires lysine 362 and alanine 364 of β-tubulin. A, alignment of β-tubulin isoforms reveals further sequence divergence between residues 349 and 371. Note the presence of Lys-362 and Ala-364 in isoforms found to bind LRRK2 (TUBB, TUBB4, and TUBB6). B, mutation S362K allows TUBB1 to bind LRRK2. C, mutation A364S in TUBB and TUBB4 abolishes the interaction of these proteins with LRRK2. By contrast, the reciprocal S364A mutation in TUBB2A, TUBB2B, TUBB2C, and TUBB3 allows these β-tubulins to interact with LRRK2. D, interaction of TUBB and TUBB4 with the LRRK2 RocCOR tandem domain is also abolished by phosphomimetic mutations at residue 364.