Introduction
Patients with heart failure (HF) experience a myriad of symptoms that are associated with marked distress and impaired quality of life.1–3 In light of their high symptom burden and poor prognosis, patients with symptomatic HF are an appropriate population in whom to introduce palliative care (PC).4–6 However, PC referral early in the course of symptomatic HF is a relatively new practice and few data exist that describe the nature of these encounters.7 The current study was conducted to describe the nature of outpatient PC services (i.e., type of services and care received, duration, and frequency of visits) used by patients discharged from the hospital with acute HF exacerbation, i.e., New York Heart Association (NYHA) Functional Class II–III, and to describe levels of symptom burden during the initial PC consultation and three months thereafter.
Methods
Study design, setting, and participants
This descriptive-exploratory study was conducted at a single university affiliated medical center. Participants were recruited from the inpatient setting through HF provider referrals.
Palliative care intervention
Participants met within seven days of hospital discharge with a PC specialist (e.g., physician or advanced practice nurse) who retained primary responsibility for their PC needs over three months. The PC specialist performed a comprehensive physical and psychosocial assessment, initiated discussions about advance care planning (e.g., completion of advance directives, options to take in the event of worsening health, family involvement, pain management, hydration issues, artificial nutrition, blood transfusions, advanced therapies, organ and tissue donation, and medical device donation), and worked with participants to develop a treatment plan that listed the goals of care. The treatment plan was presented to the interdisciplinary PC team during their weekly meetings, in order to support a team-based approach to providing patient-family centered care that encouraged active involvement of patients and their families in decision making involving their care.
Data collection methods
The study was approved by the appropriate institutional review board. Participants provided informed consent prior to completing the modified Edmonton Symptom Assessment Scale (ESAS),8 a nine-item, self-reported visual analog scale numerically rated from 0 (no symptom at all) to 10 (worst possible symptom) at baseline and three months, to assess ratings of each symptom at the time that the survey was completed.9 The severity of each individual item was categorized as none (0), mild (1–4), moderate (5–7), and severe (8–10). The reliability (Cronbach's α) of the ESAS for the current study was 0.86. Data on types of PC services received, the focus of care for each encounter, and medication use over three months were abstracted from the medical records.
Data analyses
Descriptive summaries of demographic and clinical data, symptom burden scores, and duration and frequency of PC encounters were computed using SPSS 18.0 (SPSS Inc., Chicago, IL). The paired Wilcoxon signed-rank test was used to compare symptom burden scores immediately after discharge and three months postdischarge. A symptom response rate (i.e., percentage of participants presenting a reduction of ≥2 points on an individual symptom of the ESAS) was computed to determine efficacy of PC in reducing symptom burden; a two-point change in individual symptom scores has been reported as clinically relevant in patients with cancer.9 Statistical significance was accepted at a two-sided α level of <0.05 for all analyses.
Results
Study participants and palliative care consultation
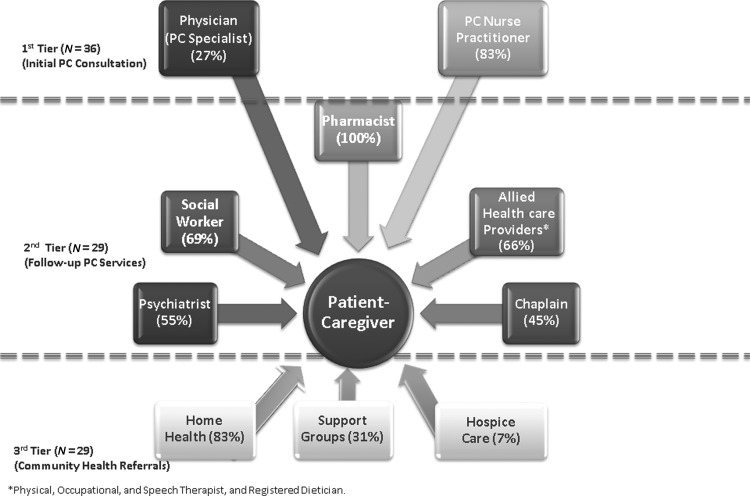
During the five-month study recruitment period, 57 patients were referred by their HF provider; 42 (73%) provided informed consent, but only 36 (85.7%) completed the initial PC consultation. All 36 patients received support for advance care planning and care coordination from their PC specialist (see Fig. 1, Tier 1). In addition, participants received support for symptom management (81%), patient education (69%), and coping (50%). The median total time for the initial PC consultation was 75 minutes (range, 50–120; quartiles=25th percentile: 60 minutes, 50th percentile: 85 minutes, 75th percentile: 100 minutes).
Fig. 1.
Palliative Care Services that participants used.
Additional palliative care services received
Following the initial PC consultation, seven (19%) felt that they did not need additional PC support. Their sociodemographic and clinical characteristics were comparable to the participants who received PC consultation plus follow-up visits (see Table 1).
Table 1.
Baseline Sociodemographic and Clinical Characteristics (N=36)
| All participants (N=36) | Initial palliative care consultation only (n=7) | Palliative care consultation + follow-up (n=29) | Sig. | |
|---|---|---|---|---|
| Age, years (mean±SD) |
53.9±8.0 |
52.7±6.3 |
54.1±8.4 |
0.850 |
| Male, n (%) |
26 (72.2) |
4 (57.1) |
22 (75.9%) |
0.583 |
| Race, n (%) |
|
|
|
0.468 |
| Hispanic |
5 (13.9) |
1 (14.3) |
4 (13.8) |
|
| White |
22 (61.1) |
3 (42.9) |
19 (55.5) |
|
| Black |
9 (25.0) |
3 (42.9) |
6 (20.7) |
|
| Married, n (%) |
25 (69.4) |
6 (85.7) |
19 (65.5) |
0.578 |
| Education, n (%) |
|
|
|
0.824 |
| ≤High school graduate |
17 (47.2) |
3 (42.8) |
14 (48.3) |
|
| Some college |
10 (27.8) |
2 (28.6) |
8 (27.6) |
|
| ≥College graduate |
9 (25.0) |
2 (28.6) |
7 (24.1) |
|
| Ejection fraction, % (mean±SD) |
25.4±5.2 |
23.1±4.3 |
25.9±5.3 |
0.094 |
| Charlson Comorbidity Index (mean±SD) |
3.7±1.5 |
2.7±1.4 |
3.9±1.5 |
0.134 |
| NYHA class, N (%) |
|
|
|
0.983 |
| Class II |
25 (69.4) |
5 (71.4) |
20 (69.0) |
|
| Class III |
11 (30.6) |
2 (28.6) |
9 (31.0) |
|
| Comorbidities | ||||
| Hypertension, n (%) |
22 (61.1) |
3 (42.9) |
19 (65.5) |
0.523 |
| Coronary artery disease, n (%) |
22 (61.1) |
4 (57.1) |
18 (62.1) |
0.957 |
| Diabetes mellitus, type 2, n (%) |
12 (33.3) |
3 (42.9) |
9 (31.0) |
0.246 |
| Overweight or obese, n (%) |
25 (69.4) |
4 (57.1) |
21 (72.4) |
0.575 |
| History smoking (previous), n (%) |
14 (38.9) |
3 (42.8) |
11 (37.9) |
0.942 |
| Medications used, N (%) | ||||
| Ace inhibitors |
26 (72.2) |
5 (71.4) |
21 (72.4) |
0.847 |
| Angiotensin receptor blockers |
7 (19.4) |
2 (28.5) |
5 (17.2) |
|
| Beta-blockers |
26 (72.2) |
5 (71.4) |
21 (72.4) |
0.847 |
| Diuretics |
23 (63.9) |
5 (71.4) |
18 (62.1) |
0.578 |
| Pain medications |
11 (30.6) |
3 (42.9) |
8 (27.6) |
0.335 |
| Antidepressants | 8 (22.2) | 2 (28.6) | 6 (20.6) | 0.430 |
NYHA, New York Heart Association.
All 29 patients who sought additional PC services were referred to the pharmacist (see Fig. 1, Tier 2) who worked with the PC specialist to determine a treatment regimen for reducing physical distress (e.g., pain) and psychosocial distress (e.g., depression). New medications were prescribed for 20 (69%) participants; 6 (30%) were prescribed opioids; 4 (20%) were prescribed antidepressants; and 10 (50%) were prescribed both. Seven (24%) required changes in their medications; 5 (71%) needed to uptitrate their pain medication and 2 (29%) were switched to a different antidepressant.
In addition, 20 (69%) sought the support of the social worker for case management and were given information related to community resources available to help them cope with their condition. Nineteen (66%) who complained of fatigue and dyspnea and 16 (55%) who complained of anxiety and depression were referred to the physical and occupational therapists and psychiatrist on the PC team, respectively, for further evaluation and treatment. Finally, 13 (45%) met with the chaplain; and 83%, 31%, and 7% were referred to home health, community support groups, and hospice, respectively.
The median number of follow-up visits for each participant over three months was two days (mean, 2.21±0.27; range, 1–4 days). The number of follow-up visits and telephone calls for the 29 patients totaled 64 and 45, respectively.
Symptom burden at baseline
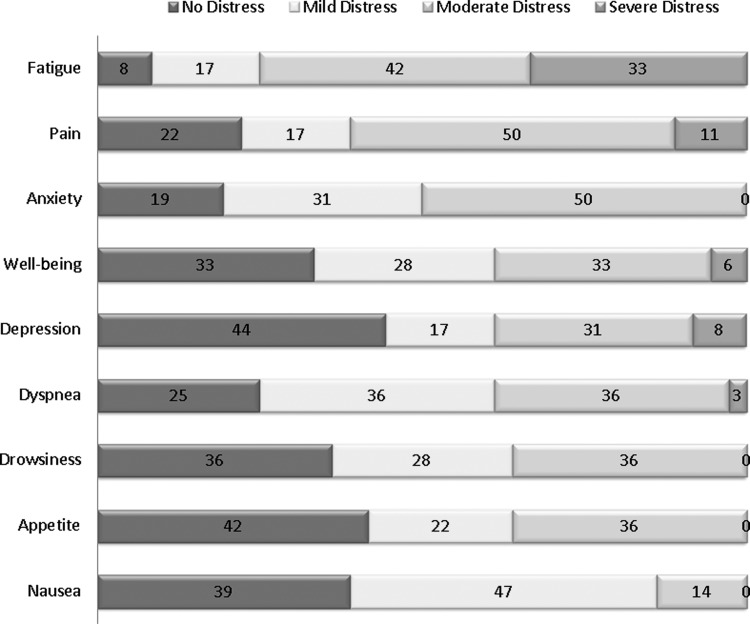
Figure 2 illustrates the proportion of patients who reported no distress versus mild, moderate, and severe distress for each of the individual symptoms at baseline. The most common symptoms were fatigue (75%), pain (61%), anxiety (50%), depression (39%), and dyspnea (39%).
Fig. 2.
Severity of Symptom Distress at baseline (N=36).
Symptom burden at three months
Table 2 reflects marked reductions in pain, anxiety, and dyspnea (all P<0.001) and moderate reductions in fatigue, depression, drowsiness, appetite, and nausea (all P≤0.040) over time. More than a fifth of the participants had an SRR (i.e., ≥2-point decrease in 0–10 score on ESAS) for anxiety (36%), pain (32%), fatigue (25%), depression (25%), dyspnea (22%), and well-being (22%). Participants who received additional PC services following the initial PC consultation were more likely to show improvements in fatigue (P<0.001), pain (P=0.044), anxiety (P=0.029), sense of well-being (P=0.035), dyspnea (P=0.008), and nausea (P=0.045) than participants who only completed the initial PC consultation. Furthermore, a greater proportion of patients who only completed the initial PC consultation showed worsening symptoms of fatigue (29% versus 11%, P<0.001) and pain (29% versus 10%, P=0.044).
Table 2.
Comparison between Baseline and Follow-up Symptom Scores (N=36)
| ESAS symptoms | Total no. of patients | SRRa n (%) | Baseline median (IQR, P25–P75) | Follow-up median (IQR, P25–P75) | Z statistic | P* |
|---|---|---|---|---|---|---|
| TSDS |
36 |
NA |
34.00 (29.00 – 40.75) |
26.50 (23.25 – 32.75) |
−6.895 |
<0.001 |
| Fatigue |
36 |
9 (25.0) |
4.50 (3.25 – 7.00) |
4.00 (3.00 – 6.00) |
−2.846 |
0.004 |
| Pain |
36 |
11 (30.6) |
6.00 (0.00 – 7.00) |
4.00 (0.00 – 6.00) |
−3.690 |
<0.001 |
| Anxiety |
36 |
13 (36.1) |
4.00 (3.00 – 6.00) |
3.50 (1.25 – 4.00) |
−3.523 |
<0.001 |
| Well-being |
36 |
8 (22.2) |
4.00 (0.00 – 6.00) |
3.00 (0.00 – 4.00) |
−4.148 |
<0.001 |
| Depression |
36 |
9 (25.0) |
4.00 (3.00 – 6.00) |
3.00 (0.00 – 5.00) |
−1.640 |
0.038 |
| Dyspnea |
36 |
8 (22.2) |
4.00 (3.00 – 5.00) |
3.00 (0.25 – 4.00) |
−3.815 |
<0.001 |
| Drowsiness |
36 |
3 (8.3) |
4.00 (0.00 – 4.00) |
3.00 (0.00 – 4.00) |
−3.231 |
0.001 |
| Appetite |
36 |
3 (8.3) |
3.00 (0.00 – 4.75) |
3.00 (0.00 – 4.00) |
−2.919 |
0.004 |
| Nausea | 36 | 4 (11.1) | 3.00 (0.00 – 4.00) | 3.00 (0.00 – 3.00) | −3.350 | 0.001 |
Defined as a two-point decrease or more in 0–10 score on individual symptoms on the ESAS; NA not analyzed.
Wilcoxon signed-rank test.
ESAS, Edmonton Symptom Assessment Scale; IQR, interquartile range; SRR, symptom response rate; TSDS, total symptom distress score.
Discussion
The current prospective study was conducted to evaluate the types of PC services used by patients recently hospitalized for acute HF exacerbation. We also present data on the prevalence of symptoms and symptom severity during the initial PC consultation and the effects of PC services on symptom burden three months postdischarge. Our data showed moderately high symptom burden, with 92% reporting at least one symptom that was mild to moderately distressful and 61% reporting at least one severely distressful symptom. This is consistent with other studies that report high levels of both physical and psychological distress in patients with symptomatic HF.1,10–14
Consistent with prior research in this area, we found that a major part of the initial PC consultation was dedicated to symptom management.4 Participants' level of symptom burden also dictated the urgency of initiating additional support from the PC team. Participants with physical and functional distress (e.g., fatigue, dyspnea) were referred for services like strength training and assistance with activities of daily living, while those with psychosocial distress (e.g., depression, anxiety) were referred for services that provided psychological support and assistance with coping. Participants with no symptoms or mild symptoms were more likely to forego additional PC support.
Earlier studies examining the effectiveness of outpatient PC services in a general medicine outpatient clinic reported improvements in dyspnea, anxiety, and spiritual well-being, but no significant impact on pain or depression.15 In contrast, we found that ongoing PC support beyond the initial PC consultation resulted in greater reductions in symptom burden, including pain and depression, confirming that on-going PC provides clinicians with the opportunity to focus on the needs and preferences of greatest importance to patients and families are more likely to result in better outcomes.16
Several important limitations must be considered when interpreting the results from our study. First, as expected with descriptive, exploratory studies, causation cannot be inferred. We cannot say that the number of PC referrals actually resulted in reduced symptom burden. Second, the generalizability of our findings is limited because the sample is from a single university affiliated medical center; patients were younger on average compared to patients with HF in the community. Third, we did not have a mechanism to verify the time spent on individual PC services during the follow-up visits. Last, our study did not have a control group and it is possible that patients who had higher symptom burden were less likely to agree to participate in the study, thus leading to underestimation of the prevalence and severity of symptoms.
Conclusion
Our data supports the growing consensus that HF patients benefit from a PC referral early in the HF disease trajectory long before they qualify for hospice care, and that ongoing care resulted in greater symptom control. Future randomized controlled trials with a larger, more heterogeneous sample are warranted to better explicate the effectiveness PC services on symptom burden and clinical outcomes (e.g., readmission, self-management, quality of life) in patients with symptomatic HF. Likewise, future investigations should also examine the type and frequency of care needed by patients and families beyond the first three months following an acute hospitalization for HF exacerbation.
Acknowledgments
The authors acknowledge funding from the National Heart, Lung, and Blood Institute (1R01HL093466-01) and the University of California, Los Angeles, Resource Centers for Minority Aging Research/Center for Health Improvement of Minority Elderly (RCMAR/CHIME) under the National Institute on Aging (P30-AG02-1684; PI, C. Mangione). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute–National Institutes of Health or the National Institute on Aging.
Author Disclosure Statement
The authors have no disclosures to make.
References
- 1.Bekelman DB, Havranek EP, Becker DM, et al. : Symptoms, depression, and quality of life in patients with heart failure. J Card Fail 2007;13(8):643–648 [DOI] [PubMed] [Google Scholar]
- 2.Bekelman DB, Rumsfeld J, Havranek EP, et al. : Symptom burden, depression, and spiritual well-being: A comparison of heart failure and advanced cancer patients. J Gen Intern Med 2009;24(5):592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambroski CH, Moser DK, Bhat G, Ziegler C: Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs 2005;4(3):198–206 [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen J, Jackson V, Dahlin C, et al. : Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med 2011;14(4):449–464 [DOI] [PubMed] [Google Scholar]
- 5.Goodlin SJ: Palliative care in congestive heart failure. JACC 2009;54(5):386–396 [DOI] [PubMed] [Google Scholar]
- 6.Goodlin SJ, Wingate S, Pressler SJ, Teerlink JR, Storey CP: Investigating pain in heart failure patients: Rationale and design of the Pain Assessment, Incidence & Nature in Heart Failure (PAIN-HF) Study. J Card Fail 2008;14(4):276–282 [DOI] [PubMed] [Google Scholar]
- 7.Brannstrom M, Boman K: A new model for integrated heart failure and palliative advanced homecare: Rationale and design of a prospective randomized study. Eur J Cardiovasc Nurs 2013;12(30):269–275 [DOI] [PubMed] [Google Scholar]
- 8.Bruera E, Kuehn N, Miller M, Selmser P, Macmillan K: The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 1991;7(2):6–9 [PubMed] [Google Scholar]
- 9.Stromgren AS, Sjogren P, Goldschmidt D, et al. : A longitudinal study of palliative care. Cancer 2005;103(8):1747–1755 [DOI] [PubMed] [Google Scholar]
- 10.Ekman I, Cleland JGF, Andersson B, Swedberg K: Exploring symptoms in chronic heart failure. Eur J Heart Fail 2005;7(5):699–703 [DOI] [PubMed] [Google Scholar]
- 11.Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL: Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage 2008;35(6):594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jurgens CY, Moser DK, Armola R, Carlson B, Sethares K, Riegel B: Symptom clusters of heart failure. Res Nurs Health 2009;32(5):551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zambroski CH, Moser DK, Bhat G, Ziegler C: Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs 2005;4(3):198–206 [DOI] [PubMed] [Google Scholar]
- 14.Ekman I, Cleland JGF, Swedberg K, Charlesworth A, Metra M, Poole-Wilson PA: Symptoms in patients with heart failure are prognostic predictors: Insights from COMET. J Card Fail 2005;11(4):288–292 [DOI] [PubMed] [Google Scholar]
- 15.Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ: The comprehensive care team: A controlled trial of outpatient palliative medicine consultation. Arch Intern Med 2004;164(1):83–91 [DOI] [PubMed] [Google Scholar]
- 16.Dev S, Abernethy AP, Rogers JG, O'Connor CM: Preferences of people with advanced heart failure: A structured narrative literature review to inform decision making in the palliative care setting. Am Heart J 2012;164(3):313–319 [DOI] [PubMed] [Google Scholar]