Abstract
In mammalian cells, reactive oxygen species (ROS) are produced via a variety of cellular oxidative processes, including the activity of NADPH oxidases (NOX), the activity of xanthine oxidases, the metabolism of arachidonic acid (AA) by lipoxygenases (LOX) and cyclooxygenases (COX), and the mitochondrial respiratory chain. Although NOX-generated ROS are the best characterized examples of ROS in mammalian cells, ROS are also generated by the oxidative metabolism (e.g., via LOX and COX) of AA that is released from the membrane phospholipids via the activity of cytosolic phospholipase A2 (cPLA2). Recently, growing evidence suggests that LOX- and COX-generated AA metabolites can induce ROS generation by stimulating NOX and that a potential signaling connection exits between the LOX/COX metabolites and NOX. In this review, we discuss the results of recent studies that report the generation of ROS by LOX metabolites, especially 5-LOX metabolites, via NOX stimulation. In particular, we have focused on the contribution of leukotriene B4 (LTB4), a potent bioactive eicosanoid that is derived from 5-LOX, and its receptors, BLT1 and BLT2, to NOX stimulation through a signaling mechanism that leads to ROS generation.
Keywords: BLT2, eicosanoids, lipoxygenase, NOX, ROS
INTRODUCTION
Reactive oxygen species (ROS) include highly reactive oxygen radicals [superoxide (O2 •-), hydroxyl (•OH), peroxyl (RO2 •), and alkoxyl (RO•)] and non-radicals that are either oxidizing agents and/or are easily converted into radicals, such as hypochlorous acid (HOCl), ozone (O3), singlet oxygen (1O2), and hydrogen peroxide (H2O2) (Bedard et al., 2007). Although the NADPH oxidase (NOX) family is well recognized as a major source of non-mitochondrial ROS in many cells, including phagocytes and non-phagocytes, arachidonic acid (AA) metabolism by lipoxygenases (LOX) and cyclooxygenases (COX) also plays a role in the generation of ROS in various cell types (Fruehauf et al., 2007; Ostuni et al., 2010). AA is released from glycerolphospholipids in the nuclear envelope and from the plasma membrane via the activity of cytosolic phospholipase A2 (cPLA2). AA is subsequently metabolized by LOX and COX to generate a variety of bioactive eicosanoids, including prostaglandins (PGs), thromboxanes (TXs), and leukotrienes (LTs) (as shown schematically in Fig. 1) (Kim et al., 2008). These enzymes generate ROS as by-products during the oxidation of AA (Yun et al., 2010). The oxidized metabolites that are generated by LOX or COX induce changes in the intracellular redox balance and have been implicated in the regulation of intracellular signaling events, such as cell proliferation, survival, and migration, and in the progression of the pathogenesis of various human diseases (de Carvalho et al., 2008; Thornber et al., 2009). Recently, growing evidence indicates that NOX is stimulated by AA metabolites that are generated by COX and LOX. Such evidence suggests a signaling connection between COX/LOX metabolites and NOX. In the present review, we focus on these recent insights into the induced generation of ROS via the AA-metabolite stimulation of NOX.
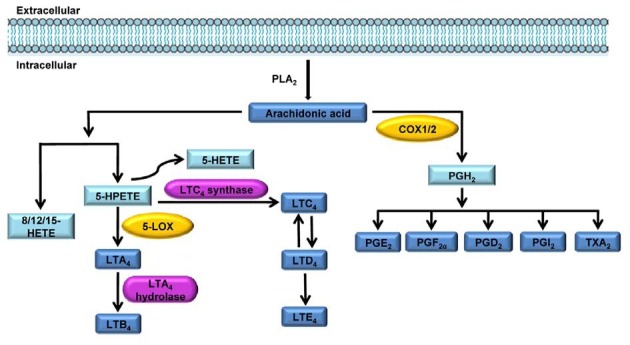
Fig. 1. Eicosanoid synthetic pathways. LOXs convert AA into biologically active metabolites such as LTs and HETEs. In the 5-LOX pathway, AA is converted to an intermediary 5-HPETE, which is further metabolized to form the unstable LTA4. LTA4 is subsequently converted to 5- HETE, LTB4, LTC4, LTD4 and LTE4. In the COX pathway, the first step is the enzymatic conversion of AA to the intermediate PGG2, which is then reduced to an intermediate, PGH2, by the peroxidase activity of COX. PGH2 is sequentially metabolized to prostanoids, including PGs and TXs, by specific PG and TX synthases.
THE PRODUCTION OF AA METABOLITES BY COX/LOX AND THE STIMULATION OF NOX
COX enzymes catalyze the conversion of AA to prostanoids, including PGs and thromboxane A2 (TXA2). COXs have three isoforms: the constitutive isoform COX-1, and the inducible isoforms COX-2 and COX-3. In the COX pathway, the first step is the enzymatic conversion of AA to the intermediate prostaglandin G2 (PGG2). PGG2 is then reduced to the intermediate prostaglandin H2 (PGH2) by the peroxidase activity of COX. PGH2 is sequentially metabolized to prostanoids, including PGs and TXs, by specific synthases (Wang et al., 2010) (Fig. 1). The PGs exert their biological effects in an autocrine or paracrine manner by binding to the cognate cell surface receptors of the G protein-coupled receptor (GPCR) family. Recently, results from several studies suggest that COX metabolites are capable of stimulating NOX. For example, the 15-deoxy-Delta (12, 14)- prostaglandin J2 (15d-PGJ2)-mediated activation of NOX led to the generation of ROS and to the induction of apoptosis in leukemia and colorectal cancer cells (Shin et al., 2009). In addition, 15d-PGJ2 caused a transient increase in the number of ROS via NOX, which led to an anti-inflammatory effect in murine macrophages (Hong et al., 2008).
LOX enzymes are classified according to the position of the insertion of oxygen in AA as 5-, 8-, 12-, and 15-LOX. 12-LOX catalyzes the stereo-specific oxygenation of AA to form 12(S)- hydroperoxyeicosatetraenoic acid (HPETE) and 12(S)-hydroxyeicosatetraenoic acid (HETE). Three types of 12-LOX have been well characterized: platelet-type, leukocyte-type, and epidermal- type (Funk et al., 1996). Although the detailed signaling mechanisms through which 12-LOX stimulates NOX have not yet been established, several reports suggest that 12-LOX acts upstream of the NOX pathways. For example, NOX1 is activated during the spreading of colorectal cancer cells in collagen IV, and this activation is dependent on 12-LOX (de Carvalho et al., 2008). In addition, 12(S)-HETE stimulation of NOX1 mediates ROS production and migration in colon adenocarcinoma cells (Sadok et al., 2008).
Two types of 15-LOX have been characterized in humans: reticulocyte-type (15-LOX-1) and epidermal-type (15-LOX-2). 15-LOX-1 is expressed in reticulocytes and eosinophils (Kuhn et al., 1999; Wittwer et al., 2007), and 15-LOX-2 is expressed in the skin, prostate gland, lungs and cornea (Brash et al., 1997; Krieg et al., 2002). Linoleic acid (LA) is the preferred substrate for 15-LOX-1. LA is metabolized to 13S-hydroperoxyoctadeca- (9Z, 11E)-dienoic acid (13-HPODE), which is later reduced to 13S-hydroxyoctadeca-(9Z,11E)-dienoic acid (13-HODE). The 15-LOX-2, in contrast, oxygenates AA to 15(S)-HPETE, which is reduced to 15(S)-HETE (Mahipal et al., 2007). Recently, several papers have reported a correlation between 15-LOX activation and NOX stimulation. For example, the NOX-activation- mediated generation of ROS has been shown to be responsible for the 15-LOX-2 metabolite-induced apoptosis in the chronic myeloid leukemia cell line K-562 (Mahipal et al., 2007). In addition, the pretreatment of cells with diphenylene iodonium (DPI) inhibited 85% of the ROS production that was induced by 15(S)-HPETE and inhibited 76% of the ROS production that was induced by 15(S)-HETE in Jurkat cells, suggesting that 15-LOX metabolite-induced apoptosis may involve ROS generation through NOX activation (Kumar et al., 2009). The detailed signaling mechanisms of NOX stimulation by 15-LOX have not yet been fully established.
The 5-LOX catalyzes the oxygenation of AA at C-5 to form the epoxide intermediate leukotriene A4 (LTA4). The efficient utilization of endogenous AA by 5-LOX requires a helper protein that is known as 5-LOX-activating protein (FLAP) (Peters- Golden et al., 2003). LTA4 can then be hydrolyzed by LTA4 hydrolase to leukotriene B4 (LTB4) or can be conjugated with glutathione by leukotriene C4 (LTC4) synthase to yield LTC4. Then, LTC4 can be converted by γ-glutamyl transpeptidase to leukotriene D4 (LTD4). In turn, LTD4 is converted to leukotriene E4 (LTE4) by dipeptidase. LTC4, LTD4 and LTE4 are known as cysteinyl LTs (cysLTs) and interact with at least two classes of receptors (cysLT1 and cysLT2) (Singh et al., 2010). Several reports suggest a relationship between the cysLT receptor and ROS generation. For example, pretreatment with cysLT1 receptor antagonists, MK571 or montelukast, have been shown to reduce vascular ROS production, to considerably improve endothelial function, and to ameliorate atherosclerotic plaque generation in vascular smooth muscle cells (VSMC) (Becher et al., 2011). Indeed, LTC4-mediated production of ROS is an essential part of the atherosclerotic response in VSMC following angiotensin (Ang) II stimulation (Mueller et al., 2008). In addition, LTD4 mediates proliferative effects through the activation of cysLT1 and subsequent ROS production in airway smooth muscle cells (ASMC) (Fang et al., 2009; Ravasi et al., 2006).
LTB4 is one of the most potent proinflammatory mediators that promote leukocyte adherence and emigration in addition to increasing vascular protein leakage by acting mainly on leukocytes such as neutrophils and eosinophils (Mackarel et al., 2000; Steiner et al., 2001; Woo et al., 2002). Previous studies have reported that LTB4 induces ROS generation in macrophages, neutrophils, eosinophils, and other fibroblasts. In macrophages, LTB4 activates NOX through the enhanced phosphorylation and subsequent membrane translocation of p47phox, a cytosolic component of NOX, in a PKC-δ-activation-dependent manner (Serezani et al., 2005a; Woo et al., 2003; Yun et al., 2010). Similarly, LTB4 contributes to the activation of p47phox in polyunsaturated fatty acids (PUFA)-stimulated polymorphonuclear leukocytes (Serezani et al., 2005b). In addition, LTB4 plays a role in promoting the robust, receptor-mediated activation of NOX in guinea pig eosinophils (Lindsay et al., 1998a; 1998b). In addition, Woo et al. reported that LTB4 plays a crucial role in ROS-promoted chemotaxis and proliferation in fibroblasts via the Rac, ERK and cPLA2 signaling pathways (Woo et al., 2000a; 2000b; 2002).
BLT1/BLT2 AND NOX
Two GPCRs for LTB4 have been identified, BLT1 and BLT2 (Tager et al., 2003; Yokomizo et al., 1997; 2000; 2001). These GPCRs mediate the activities of LTB4 and function in host immune responses and in the pathogenesis of various inflammatory diseases. The majority of the studies on LTB4 receptors have focused on the high-affinity receptor, BLT1. The mediatory role of ROS production via BLT1 has been demonstrated using synthetic BLT1 antagonists. For example, the BLT1 receptor antagonist LY293111 inhibits the proliferation of anapla-stic large cell lymphoma (ALCL) cells by arresting the cells in the G1 phase of the cell cycle and by inducing ROS-mediated apoptosis (Zhang et al., 2005). Reduced phagocytosis has also been observed in human neutrophils that were pretreated with a LTB4 receptor antagonist (LY292476), implicating LTB4 in neutrophil phagocytic activity (Tager et al., 2003). Pretreatment with a BLT1 antagonist (CP105696) has been shown to decrease the phosphorylation and translocation of p47phox in AAstimulated polymorphonuclear leukocytes (Serezani et al., 2005b).
BLT2, a low-affinity LTB4 receptor, is expressed in a wide variety of tissues (Yokomizo et al., 2000). Although no clear physiological function has been identified for BLT2, recent reports suggest that BLT2 plays a key role in ROS generation. LTB4-induced ROS generation was completely abolished by the inhibition of BLT2 expression in Rat2 fibroblasts (Woo et al., 2000a). Recently, several reports have demonstrated that BLT2 is critical for NOX upregulation. For example, the induced expression of BLT2 by oncogenic H-Ras results in the generation of ROS via NOX1 upregulation (Choi et al., 2008). Previous studies have also demonstrated that the LTB4-BLT2-NOX1- linked cascade plays a critical role in the invasive and metastatic activity that is associated with oncogenic H-Ras (Choi et al., 2008; Kim et al., 2010a). In addition, increased ROS generation appears to play an important role in maintaining cancer phenotypes due to the stimulating effects of ROS on cell growth and proliferation (Hu et al., 2005). Very recently, we demonstrated that NOX1-derived ROS generation is an essential downstream component of the BLT2-mediated pro-survival signaling that protects cells from apoptotic death in estrogen (ER)-negative breast cancer cells (Choi et al., 2010). Additionally, in aggressive bladder cancer cells, BLT2 was shown to promote an invasive, metastatic phenotype by stimulating the NOX1/4- dependent generation of ROS (Kim et al., 2010b).
Ryu et al. demonstrated that the BLT2 pathway is responsible for the upregulation of NOX1 and subsequent ROS generation in response to UVB irradiation. A blockade of BLT2 with the BLT2 inhibitor LY255283 or with siBLT2 attenuated NOX1- mediated ROS production and subsequently reduced the detected apoptotic cell death in HaCaT cells and in primary human keratinocytes (Kim et al., 2010c; Ryu et al., 2010). The BLT2-mediated NOX1 upregulation-dependent pathway has also been shown to promote the secretion of IL-8 from human mast cells in response to the pro-inflammatory cytokine IL- 1beta, contributing to angiogenesis (Kim et al., 2010d). Another study indicated that BLT2-stimulated NOX1 upregulation is a downstream signaling route that mediates the synthesis of Th2 cytokines (IL-4 and IL-13) in antigen-stimulated bone marrowderived mast cells (Cho et al., 2010). The mechanism through which BLT2 mediates NOX upregulation has not yet been clearly demonstrated. Future studies of the LTB4 receptors that mediate ROS generation and their relationships to NOX will enhance our understanding of the signaling pathways that are involved in ROS generation.
CONCLUSION
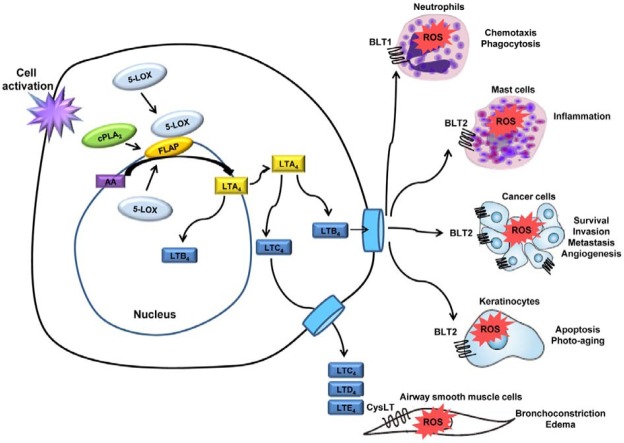
There is a growing body of evidence to suggest that 5-LOXderived ROS are involved in a variety of pathological and inflammatory responses. However, the detailed signaling mechanisms through which LOX metabolites mediate the specific signaling pathways that are involved in ROS generation, especially through NOX upregulation, have yet to be clearly demonstrated. As shown in Fig. 2, the stimulation of cells through their receptors likely induces the production of ROS by NOX upregulation, and these endogenously produced oxidants have important functions in the regulation of various responses, including chemotaxis, phagocytosis, inflammation response, cancer progression, apoptosis and aging. Further studies are necessary to fully characterize the relationship between eicosanoid receptors and NOX upregulation in terms of ROS signaling.
Fig. 2. The leukotriene-ROS linked cascade in various cell types. The stimulation of various cells through their receptors induces the production of ROS via NOX upregulation. These endogenously produced oxidants have important functions in the regulation of various responses, including chemotaxis, phagocytosis, inflammation response, cancer progression, apoptosis and aging.
References
- 1.Becher U.M., Ghanem A., Tiyerili V., Furst D.O., Nickenig G., Mueller C.F. Inhibition of leukotriene C(4) action reduces oxidative stress and apoptosis in cardiomyocytes and impedes remodeling after myocardial injury. J. Mol. Cell Cardiol. (2011);50:570–577. doi: 10.1016/j.yjmcc.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Bedard K., Krause K.H. The NOX family of ROSgenerating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. (2007);87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 3.Brash A.R., Boeglin W.E., Chang M.S. Discovery of a second 15S-lipoxygenase in humans. Proc. Natl. Acad. Sci. USA. (1997);94:6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho K.J., Seo J.M., Lee M.G., Kim J.H. BLT2 Is upregulated in allergen-stimulated mast cells and mediates the synthesis of Th2 cytokines. J. Immunol. (2010);185:6329–6337. doi: 10.4049/jimmunol.1001213. [DOI] [PubMed] [Google Scholar]
- 5.Choi J.A., Kim E.Y., Song H., Kim C., Kim J.H. Reac-tive oxygen species are generated through a BLT2-linked cascade in Ras-transformed cells. Free Radic. Biol. Med. (2008);44:624–634. doi: 10.1016/j.freeradbiomed.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 6.Choi J.A., Lee J.W., Kim H., Kim E.Y., Seo J.M., Ko J., Kim J.H. Pro-survival of estrogen receptor-negative breast cancer cells is regulated by a BLT2-reactive oxygen specieslinked signaling pathway. Carcinogenesis. (2010);31:543–551. doi: 10.1093/carcin/bgp203. [DOI] [PubMed] [Google Scholar]
- 7.de Carvalho D.D., Sadok A., Bourgarel-Rey V., Gattacceca F., Penel C., Lehmann M., Kovacic H. Nox1 downstream of 12-lipoxygenase controls cell proliferation but not cell spreading of colon cancer cells. Int. J. Cancer. (2008);122:1757–1764. doi: 10.1002/ijc.23300. [DOI] [PubMed] [Google Scholar]
- 8.Fang S.H., Yuan Y.M., Peng F., Li C.T., Zhang L.H., Lu Y.B., Zhang W.P., Wei E.Q. Pranlukast attenuates ischemia-like injury in endothelial cells via inhibiting reactive oxygen species production and nuclear factor-kappaB activation. J. Cardiovasc. Pharmacol. (2009);53:77–85. doi: 10.1097/FJC.0b013e318196736c. [DOI] [PubMed] [Google Scholar]
- 9.Fruehauf J.P., Meyskens F.L., Jr. Reactive oxygen species: a breath of life or death? Clin. Cancer Res. (2007);13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 10.Funk C.D., Keeney D.S., Oliw E.H., Boeglin W.E., Brash A.R. Functional expression and cellular localization of a mouse epidermal lipoxygenase. J. Biol. Chem. (1996);271:23338–23344. doi: 10.1074/jbc.271.38.23338. [DOI] [PubMed] [Google Scholar]
- 11.Hong H.Y., Jeon W.K., Kim B.C. Up-regulation of heme oxygenase-1 expression through the Rac1/NADPH oxidase/ ROS/p38 signaling cascade mediates the anti-inflammatory effect of 15-deoxy-delta 12,14-prostaglandin J2 in murine macrophages. FEBS Lett. (2008);582:861–868. doi: 10.1016/j.febslet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y., Rosen D.G., Zhou Y., Feng L., Yang G., Liu J., Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J. Biol. Chem. (2005);280:39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- 13.Kim C., Kim J.Y., Kim J.H. Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species. BMB Rep. (2008);41:555–559. doi: 10.5483/bmbrep.2008.41.8.555. [DOI] [PubMed] [Google Scholar]
- 14.Kim E.Y., Seo J.M., Cho K.J., Kim J.H. Ras-induced invasion and metastasis are regulated by a leukotriene B4 receptor BLT2-linked pathway. Oncogene. (2010a);29:1167–1178. doi: 10.1038/onc.2009.412. [DOI] [PubMed] [Google Scholar]
- 15.Kim E.Y., Seo J.M., Kim C., Lee J.E., Lee K.M., Kim J.H. BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen specieslinked pathway. Free Radic. Biol. Med. (2010b);49:1072–1081. doi: 10.1016/j.freeradbiomed.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Kim C., Ryu H.C., Kim J.H. Low-dose UVB irradiation stimulates matrix metalloproteinase-1 expression via a BLT2-linked pathway in HaCaT cells. Exp. Mol. Med. (2010c);42:833–841. doi: 10.3858/emm.2010.42.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim G.Y., Lee J.W., Ryu H.C., Wei J.D., Seong C.M., Kim J.H. Proinflammatory cytokine IL-1beta stimulates IL-8 synthesis in mast cells via a leukotriene B4 receptor 2-linked pathway, contributing to angiogenesis. J. Immunol. (2010d);184:3946–3954. doi: 10.4049/jimmunol.0901735. [DOI] [PubMed] [Google Scholar]
- 18.Krieg P., Heidt M., Siebert M., Kinzig A., Marks F., Furstenberger G. Epidermis-type lipoxygenases. Adv. Exp. Med. Biol. (2002);507:165–170. doi: 10.1007/978-1-4615-0193-0_26. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn H., Thiele B.J. The diversity of the lipoxygenase family. Many sequence data but little information on biological significance. FEBS Lett. (1999);449:7–11. doi: 10.1016/s0014-5793(99)00396-8. [DOI] [PubMed] [Google Scholar]
- 20.Kumar K.A., Arunasree K.M., Roy K.R., Reddy N.P., Aparna A., Reddy G.V., Reddanna P. Effects of (15S)-hydroperoxyeicosatetraenoic acid and (15S)-hydroxyeicosatetraenoic acid on the acute- lymphoblastic-leukaemia cell line Jurkat: activation of the Fas-mediated death pathway. Biotechnol. Appl. Biochem. (2009);52:121–133. doi: 10.1042/BA20070264. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay M.A., Haddad E.B., Rousell J., Teixeira M.M., Hellewell P.G., Barnes P.J., Giembycz M.A. Role of the mitogen-activated protein kinases and tyrosine kinases during leukotriene B4-induced eosinophil activation. J. Leukoc. Biol. (1998a);64:555–562. doi: 10.1002/jlb.64.4.555. [DOI] [PubMed] [Google Scholar]
- 22.Lindsay M.A., Perkins R.S., Barnes P.J., Giembycz M.A. Leukotriene B4 activates the NADPH oxidase in eosinophils by a pertussis toxin-sensitive mechanism that is largely independent of arachidonic acid mobilization. J. Immunol. (1998b);160:4526–4534. [PubMed] [Google Scholar]
- 23.Mackarel A.J., Russell K.J., Brady C.S., FitzGerald M.X., O’Connor C.M. Interleukin-8 and leukotriene-B(4), but not formylmethionyl leucylphenylalanine, stimulate CD18-independent migration of neutrophils across human pulmonary endothelial cells in vitro. Am. J. Respir. Cell Mol. Biol. (2000);23:154–161. doi: 10.1165/ajrcmb.23.2.3853. [DOI] [PubMed] [Google Scholar]
- 24.Mahipal S.V., Subhashini J., Reddy M.C., Reddy M.M., Anilkumar K., Roy K.R., Reddy G.V., Reddanna P. Effect of 15-lipoxygenase metabolites, 15-(S)-HPETE and 15-(S)- HETE on chronic myelogenous leukemia cell line K-562: reactive oxygen species (ROS) mediate caspase-dependent apoptosis. Biochem. Pharmacol. (2007);74:202–214. doi: 10.1016/j.bcp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Mueller C.F., Wassmann K., Widder J.D., Wassmann S., Chen C.H., Keuler B., Kudin A., Kunz W.S., Nickenig G. Multidrug resistance protein-1 affects oxidative stress, endothelial dysfunction, and atherogenesis via leukotriene C4 export. Circulation. (2008);117:2912–2918. doi: 10.1161/CIRCULATIONAHA.107.747667. [DOI] [PubMed] [Google Scholar]
- 26.Ostuni M.A., Gelinotte M., Bizouarn T., Baciou L., Houee- Levin C. Targeting NADPH-oxidase by reactive oxygen species reveals an initial sensitive step in the assembly process. Free Radic. Biol. Med. (2010);49:900–907. doi: 10.1016/j.freeradbiomed.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Peters-Golden M., Brock T.G. 5-lipoxygenase and FLAP. Prostaglandins Leukot. Essent. Fatty Acids. (2003);69:99–109. doi: 10.1016/s0952-3278(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 28.Ravasi S., Citro S., Viviani B., Capra V., Rovati G.E. CysLT1 receptor-induced human airway smooth muscle cells proliferation requires ROS generation, EGF receptor transactivation and ERK1/2 phosphorylation. Respir. Res. (2006);7:42. doi: 10.1186/1465-9921-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryu H.C., Kim C., Kim J.Y., Chung J.H., Kim J.H. UVB radiation induces apoptosis in keratinocytes by activating a pathway linked to “BLT2-reactive oxygen species”. J. Invest. Dermatol. (2010);130:1095–1106. doi: 10.1038/jid.2009.436. [DOI] [PubMed] [Google Scholar]
- 30.Sadok A., Bourgarel-Rey V., Gattacceca F., Penel C., Lehmann M., Kovacic H. Nox1-dependent superoxide production controls colon adenocarcinoma cell migration. Biochim. Biophys. Acta. (2008);1783:23–33. doi: 10.1016/j.bbamcr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Serezani C.H., Aronoff D.M., Jancar S., Mancuso P., Peters- Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. (2005a);106:1067–1075. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serezani C.H., Aronoff D.M., Jancar S., Peters-Golden M. Leukotriene B4 mediates p47phox phosphorylation and membrane translocation in polyunsaturated fatty acid-stimulated neutrophils. J. Leukoc. Biol. (2005b);78:976–984. doi: 10.1189/jlb.1004587. [DOI] [PubMed] [Google Scholar]
- 33.Shin S.W., Seo C.Y., Han H., Han J.Y., Jeong J.S., Kwak J.Y., Park J.I. 15d-PGJ2 induces apoptosis by reactive oxygen species-mediated inactivation of Akt in leukemia and colorectal cancer cells and shows in vivo antitumor activity. Clin. Cancer Res. (2009);15:5414–5425. doi: 10.1158/1078-0432.CCR-08-3101. [DOI] [PubMed] [Google Scholar]
- 34.Singh R.K., Gupta S., Dastidar S., Ray A. Cysteinyl leukotrienes and their receptors: molecular and functional characteristics. Pharmacology. (2009);85:336–349. doi: 10.1159/000312669. [DOI] [PubMed] [Google Scholar]
- 35.Steiner D.R., Gonzalez N.C., Wood J.G. Leukotriene B(4) promotes reactive oxidant generation and leukocyte adherence during acute hypoxia. J. Appl. Physiol. (2001);91:1160–1167. doi: 10.1152/jappl.2001.91.3.1160. [DOI] [PubMed] [Google Scholar]
- 36.Tager A.M., Luster A.D. BLT1 and BLT2: the leukotriene B(4) receptors. Prostaglandins Leukot. Essent. Fatty Acids. (2003);69:123–134. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 37.Thornber K., Colomba A., Ceccato L., Delsol G., Payrastre B., Gaits-Iacovoni F. Reactive oxygen species and lipoxygenases regulate the oncogenicity of NPM-ALK-positive anaplastic large cell lymphomas. Oncogene. (2009);28:2690–2696. doi: 10.1038/onc.2009.125. [DOI] [PubMed] [Google Scholar]
- 38.Wang D., Dubois R.N. Eicosanoids and cancer. Nat. Rev. Cancer. (2010);10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wittwer J., Hersberger M. The two faces of the 15- lipoxygenase in atherosclerosis. Prostaglandins Leukot. Essent. Fatty Acids. (2007);77:67–77. doi: 10.1016/j.plefa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Woo C.H., Eom Y.W., Yoo M.H., You H.J., Han H.J., Song W.K., Yoo Y.J., Chun J.S., Kim J.H. Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J. Biol. Chem. (2000a);275:32357–32362. doi: 10.1074/jbc.M005638200. [DOI] [PubMed] [Google Scholar]
- 41.Woo C.H., Lee Z.W., Kim B.C., Ha K.S., Kim J.H. Involvement of cytosolic phospholipase A2, and the subsequent release of arachidonic acid, in signalling by rac for the generation of intracellular reactive oxygen species in rat-2 fibroblasts. Biochem. J. (2000b);348(Pt 3):525–530. [PMC free article] [PubMed] [Google Scholar]
- 42.Woo C.H., You H.J., Cho S.H., Eom Y.W., Chun J.S., Yoo Y.J., Kim J.H. Leukotriene B(4) stimulates Rac-ERK cascade to generate reactive oxygen species that mediates chemotaxis. J. Biol. Chem. (2002);277:8572–8578. doi: 10.1074/jbc.M104766200. [DOI] [PubMed] [Google Scholar]
- 43.Woo C.H., Yoo M.H., You H.J., Cho S.H., Mun Y.C., Seong C.M., Kim J.H. Transepithelial migration of neutrophils in response to leukotriene B4 is mediated by a reactive oxygen species-extracellular signal-regulated kinase-linked cascade. J. Immunol. (2003);170:6273–6279. doi: 10.4049/jimmunol.170.12.6273. [DOI] [PubMed] [Google Scholar]
- 44.Yokomizo T., Izumi T., Chang K., Takuwa Y., Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. (1997);387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 45.Yokomizo T., Kato K., Terawaki K., Izumi T., Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J. Exp. Med. (2000);192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yokomizo T., Kato K., Hagiya H., Izumi T., Shimizu T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J. Biol. Chem. (2001);276:12454–12459. doi: 10.1074/jbc.M011361200. [DOI] [PubMed] [Google Scholar]
- 47.Yun M.R., Park H.M., Seo K.W., Lee S.J., Im D.S., Kim C.D. 5-Lipoxygenase plays an essential role in 4-HNE-enhanced ROS production in murine macrophages via activation of NADPH oxidase. Free Radic. Res. (2010);44:742–750. doi: 10.3109/10715761003758122. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W., McQueen T., Schober W., Rassidakis G., Andreeff M., Konopleva M. Leukotriene B4 receptor inhibitor LY293111 induces cell cycle arrest and apoptosis in human anaplastic large-cell lymphoma cells via JNK phosphorylation. Leukemia. (2005);19:1977–1984. doi: 10.1038/sj.leu.2403929. [DOI] [PubMed] [Google Scholar]