Abstract
1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG), a polyphenolic compound isolated from Rhus chinensis Mill. PGG has been known to have anti-tumor, anti-angiogenic and anti-diabetic activities. The present study revealed another underlying molecular target of PGG in MDA-MB-231 breast cancer cells by using Illumina Human Ref-8 expression BeadChip assay. Through the Beadstudio v3 micro assay program to compare the identified genes expressed in PGG-treated MDA-MB-231 cells with untreated control, we found several unique genes that are closely associated with pyruvate metabolism, glycolysis/gluconeogenesis and tyrosine metabolism, including PC, ACSS2, ACACA, ACYP2, ALDH3B1, FBP1, PRMT2 and COMT. Consistent with microarray data, real-time RT-PCR confirmed the significant down-regulation of these genes at mRNA level in PGG-treated MDA-MB-231 cells. Our findings suggest the potential of PGG as anticancer agent for breast cancer cells by targeting cancer metabolism genes.
Keywords: cancer metabolism, MDA-MB-231, microarray, PGG, real-time PCR
INTRODUCTION
Recently many medicinal herbs are attractive due to their anticancer activity with little side effects in various types of cancer. 1,2,3,4,6-penta-O-galloyl-β-D-glucose (PGG) (Fig. 1) is a water soluble gallotannin polyphenolic compound (Hofmann and Gross, 1990) isolated from gallnut of Rhus chinensis Mill, Acer truncatum Bunge, Pelargonium inquinans Ait, and Paeonia lactiflora Pall exhibiting anti-tumor, anti-angiogenesis and antidiabetic activities (Huh et al., 2005; 2008; 2009a; 2009b; Lee et al., 2004; Li et al., 2005; Oh et al., 2001; Pan et al., 1999; Zhang et al., 2009). PGG induced apoptosis through caspase-3 activation in human leukemia cells (Pan et al., 1999) and mediated cell cycle arrest at the G1 phase by targeting cyclin D1 in human hepatocellular carcinoma and prostate cancer cells (Hu et al., 2009b; Oh et al., 2001). Also, PGG efficiently blocked VEGF-induced proliferation of human umbilical vein endothelial cells (HUVECs) and the growth of immortalized human microvascular endothelial cells (HMECs) (Lee et al., 2004). In addition, PGG decreased blood glucose levels and improved glucose tolerance in diabetic and obese animals (Li et al., 2005).
Fig. 1. Chemical structure of 1,2,3,4,6-penta-O-galloyl-β-D-glucose (PGG). Molecular weight = 940.
Despite current knowledge about the multi-biological activities of PGG, the underlying mechanisms of PGG still remain unclear. In the present study, systemic biological activities of PGG were evaluated in breast cancer cell lines by microarray analysis. We have found that novel effect of PGG in MDA-MB- 231 breast cells was characterized by a striking reduction of metabolic genes, including those involved in pyruvate metabolism, glycolysis/gluconeogenesis and tyrosine metabolisms. Real-time RT-PCR also provided the similar results.
MATERIALS AND METHODS
Cell culture
MDA-MB-231 breast cancer cells were obtained from the American Type Culture Collection (ATCC) (USA) and maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 2 μm L-glutamine and penicillin/streptomycin.
Isolation and identification of 1,2,3,4,6-penta-O-galloyl-β-Dglucose (PGG)
Gallnut of R. chinensis Mill was obtained from the Oriental Medical Hospital of Kyung Hee University in Seoul, and kindly authenticated by professor Namin Baek in the Department of Oriental Herbal Materials, Kyung Hee University. The methanol extract (252 g) was dissolved in distilled water (800 ml) and successively fractionated with equal volumes of n-hexane, ethyl acetate and butanol with water; the resulting butanol fraction (35 g) was subjected to silica gel column chromatography and eluted by chloroform, methanol and H2O (65:35:10) and ethyl acetate, methanol and H2O (100:15.6:13.5), followed by purification using HPLC (J’sphere ODS-HP80, 250 × 20 mm ID, S-4 μm, 80A, ethyl acetate:methanol:H2O = 6:3:1). The active compound was identified as PGG (MW = 940; Fig. 1A) by NMR (Varian UNITY INOVA 500 NMR spectrometer, USA) and FABMS (JEOL JMS700 mass spectrometrty, USA) analysis with a purity of N98% (Huh et al., 2005).
Cytotoxicity assay
Cytotoxic effect of PGG was evaluated against MDA-MB-231 cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were inoculated onto 96-well flat bottom microplates and incubated for 24 h to allow attachment to the microtiter plates and incubated with various concentrations of PGG (0, 5, 10, 20 or 40 μM). After continuous exposure to the compounds for 24 h, MTT (5 mg/ml) was added onto each wells, then incubated until formazan was constituted. After medium was removed, formazan was dissolved with dimethyl sulfoxide (DMSO) and then measured OD values using microplate reader (Molecular Devices Co., USA) at 450 nm. Cell viability was calculated as a percentage of viable cells in PGGtreated cells versus untreated control by following equation. Cell viability (%) = [OD (PGG) - OD (Blank)] / [OD (Control) - OD (Blank)] × 100. Each experiment was repeated three times.
Total RNA isolation
Total RNA was isolated using the RNeasy Mini kit (Qiagen, Germany) following the manufacturer’s instructions.
Labeling and purification
Total RNA was amplified and purified using the Ambion Illumina RNA amplification kit (Ambion, USA) to yield biotinylated cRNA according to the manufacturer’s instructions. Briefly, 550 ng of total RNA was reverse-transcribed to cDNA using a T7 oligo (dT) primer. Second-strand cDNA was synthesized, in vitro transcribed, and labeled with biotin-NTP. After purification, the cRNA was quantified using the ND-1000 Spectrophotometer (NanoDrop, USA).
Hybridization and data export
Illumina Human Ref-8 expression BeadChip (P/N BD-25-203, Illumina Inc., Ambion, USA) arrays were used in this study. 750 ng of labeled cRNA samples were hybridized to each human-8 expression bead array for 16-18 h at 58℃, according to the manufacturer’s instructions. Detection of array signal was carried out using Amersham fluorolink streptavidin-Cy3 (GE Healthcare Bio-Sciences, UK) following the bead array manual. Arrays were scanned with an Illumina bead array reader confocal scanner according to the manufacturer’s instructions. Array data export processing and analysis was performed using Illumina GenomeStudio v2009.2 (Gene Expression Module v1.5.4).
Data analysis
The Beadstudio v3.0 was used to evaluate the expression signals generated by the Illumina Human Ref-8 expression Bead- Chip array. The 1.5-fold differentially expressed genes (DEGs) were clustered using GenPlex™ v3.0 software (ISTECH Inc., Korea). Gene ontology classification was offered by the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
Real-time RT-PCR analysis
To verify microarray results, real-time RT-PCR analysis was performed for selected genes with an Applied Biosystems 7300 Real-time PCR system using the SYBR green fluorescence quantification system (Applied Biosystems, USA) to quantify the amplicons. The PCR conditions were 40 cycles of 95℃ (15 s), 60℃ (1 min), and a standard denaturation curve. Primer sequences are listed in the 5′ to 3′ orientation in Table 1.
Table 1.
Primers used in the real-time PCR
| Target genes | Primer sequences (5′ → 3′) | |
|---|---|---|
| COMT | S | GTA CTG AAG GTG CCA GA |
| AS | GTT GCA GTT CAG AGA GG | |
| ALDH3B1 | S | GCC CAA TTT CCT AAC AAG CC |
| AS | TGT CCC TCT TCC CGA CTA AA | |
| FBP1 | S | TCC CAC AGA CAT TCA CCA GA |
| AS | AAG GTC CAG GTA GAG GCA AT | |
| ACACA | S | TTG GAG GCA CAG AAC ATG AG |
| AS | ATA ATA TGG CGG TCT CCG TC | |
| ACSS2 | S | AGC TTG TCT TCC TTG TCC TC |
| AS | TAT TCC TAC AAC CAC AGG GC | |
| PRMT2 | S | CGG AGC TCC ATG TTC CTA AG |
| AS | GGC AGT AAG GGC ACC ACT AA | |
| PC | S | ATT CCT TTC AGC CAT CGT CC |
| AS | AGA TAG GAC CCC TAA ACC TCC | |
| ACYP2 | S | AGT CAC TCA AAT CCG TGG AC |
| AS | GGT GCC TTT GCT GGT ATT CT | |
| ACTB | S | CCG AGG ACT TTG ATT GCA CA |
| AS | ACT GGG CCA TTC TCC TTA GA | |
Western blotting
MDA-MB-231 cells were lyzed in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, and protease inhibitors cocktail). The extracts were incubated on ice for 30 min and supernatants were collected by centrifugation at 14,000 × g at 4℃. Proteins in the supernatants were quantified by using a Bio- Rad DC protein assay kit II (Bio-Rad, USA), separated on 12.5% SDS-PAGE gel and electrotransferred onto a Hybond ECL transfer membrane. The membranes were blocked in 5% nonfat skim milk and probed with primary antibodies for PDK3, PGM3 (Santa Cruz Biotechnology, USA) and β-actin (Sigma Aldrich, USA), and horseradish peroxidase (HRP)-conjugated secondary antibodies. Protein expressions were detected by using enhanced chemiluminescence (ECL) (Amersham Pharmacia, USA) system.
Statistical analysis
All data were presented as mean ± standard deviation (S.D). Statistical significance was verified by Student’s t-test using Sigmaplot software (Systat Software Inc., USA).
RESULTS
Cytotoxic effect of PGG against MDA-MB-231 cells
To examine cytotoxic effect of PGG against MDA-MB-231 breast cancer cell line, MTT assay was performed. Cells were exposed to various concentrations of PGG (0, 5, 10, 20 or 40 μM) for 24 h. As shown in Fig. 2, cell viability was slightly decreased in a concentration-dependent manner and maintained up to ~70% at 40 μM of PGG.
Fig. 2. Cytotoxic effect of PGG in MDA-MB-231 cells. MDA-MB-231 cells were treated with various concentrations of PGG (0, 5, 10, 20 or 40 μM) for 24 h. Cell viability was determined by MTT assay. Data represent means ± SD.
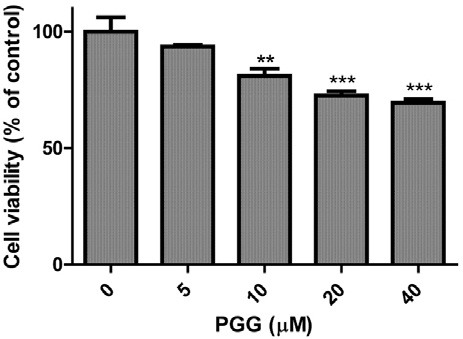
Table 2.
Up-regulation of genes based on comparison of gene expression between control (untreated) and experimental (PGG-treated) MDAMB- 231 cells
| Genes | Accession no. | Symbol | Fold change* | p-value |
|---|---|---|---|---|
| JAK/STAT signaling pathway | ||||
| Cardiotrophin-like cytokine factor 1 | NM_013246.2 | CLCF1 | 3.75 | 0.02 |
| Colony stimulating factor 2 | NM_000758.2 | CSF2 | 7.20 | 0.02 |
| Interleukin 12A | NM_000882.2 | IL12A | 2.67 | 0.03 |
| Interleukin 15 | NM_172174.1 | IL15 | 2.05 | 0.01 |
| Interleukin 24 | NM_006850.2 | IL24 | 5.52 | 0.00 |
| Interleukin 11 | NM_000641.2 | IL11 | 4.12 | 0.00 |
| Pim-1 oncogene | NM_002648.2 | PIM1 | 2.48 | 0.03 |
| Cytokine-cytokine receptor interaction | ||||
| Chemokine (C-C motif) ligand 20 | NM_004591.1 | CCL20 | 3.25 | 0.01 |
| Cardiotrophin-like cytokine factor 1 | NM_013246.2 | CLCF1 | 3.75 | 0.02 |
| Colony stimulating factor 2 | NM_000758.2 | CSF2 | 7.20 | 0.02 |
| Interleukin 12A | NM_000882.2 | IL12A | 2.67 | 0.03 |
| Bone morphogenetic protein 2 | NM_001200.1 | BMP2 | 2.51 | 0.00 |
| Interleukin 24 | NM_006850.2 | IL24 | 5.52 | 0.01 |
| Interleukin 11 | NM_000641.2 | IL11 | 4.12 | 0.00 |
| Epidermal growth factor receptor | NM_005228.3 | EGFR | 2.88 | 0.01 |
| Interleukin 1 receptor accessory protein | NM_134470.2 | IL1RAP | 2.13 | 0.00 |
| ErbB signaling pathway | ||||
| V-abl Abelson murine leukemia viral oncogene homolog 2 | NM_005158.2 | ABL2 | 3.13 | 0.01 |
| Glycogen synthase kinase 3 beta | NM_002093.2 | GSK3B | 2.08 | 0.00 |
| Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | NM_078467.1 | CDKN1A | 2.67 | 0.00 |
| Epidermal growth factor receptor | NM_005228.3 | EGFR | 2.88 | 0.01 |
| Jun oncogene | NM_002228.3 | JUN | 2.02 | 0.02 |
| Transforming growth factor, alpha | NM_003236.1 | TGFA | 2.48 | 0.00 |
| Heparin-binding EGF-like growth factor | NM_001945.1 | HBEGF | 2.18 | 0.00 |
| MAPK signaling pathway | ||||
| Dual specificity phosphatase 5 | NM_004419.3 | DUSP5 | 3.19 | 0.00 |
| Protein tyrosine phosphatase, receptor type, R | NM_002849.2 | PTPRR | 2.73 | 0.01 |
| Protein phosphatase 3 (formerly 2B), catalytic subunit, gamma isoform | NM_005605.3 | PPP3CC | 3.27 | 0.00 |
| Mitogen-activated protein kinase 7 | NM_139034.1 | MAPK7 | 2.28 | 0.00 |
| Epidermal growth factor receptor | NM_005228.3 | EGFR | 2.88 | 0.01 |
| Jun oncogene | NM_002228.3 | JUN | 2.02 | 0.02 |
| Growth arrest and DNA-damage-inducible, alpha | NM_001924.2 | GADD45A | 3.37 | 0.00 |
| VEGF signaling | ||||
| Protein phosphatase 3 (formerly 2B) | NM_005605.3 | PPP3CC | 3.27 | 0.00 |
| Prostaglandin-endoperoxide synthase 2 | NM_000963.1 | PTGS2 | 3.36 | 0.04 |
| Sphingosine kinase 1 | NM_021972.2 | SPHK1 | 2.60 | 0.00 |
Gene expression profiles in PGG-treated MDA-MB-231 cells
Gene expression profiles were significantly up- or downregulated in PGG-treated MDA-MB-231 cells compared with untreated control. Total 624 genes were differentially expressed (307 up-regulated and 317 down-regulated genes). Gene ontology annotation was achieved using the KEGG database and the genes were placed into 30 biological processes; signal transduction (15.3%), nucleoside, nucleotide and nucleic acid metabolism (11.7%), protein metabolism and modification (11.4%), immunity and defense (8.3%), developmental processes (8.2%), cell cycle (6.5%), transport (4%) and lipid, fatty acid and steroid metabolism (4%), and 27 molecular functions; nucleic acid binding (13%), select regulatory molecule (9.7%), transcription factor (7.3%), transferase (6.7%), receptor (5.6%), kinase (5.2%), signaling molecule (5.1%) and oxidoreductase (5.1%) (Fig. 3). Functionally annotated 46 genes were listed in Tables 2 and 3, which reveals a comparison of the expression levels for a variety of genes between untreated and PGG-treated groups.
Fig. 3. Gene ontology assignment of differentially expressed genes. Clustergram of up- and down-regulated genes in MDA-MB-231 cells. Microarray data from control (untreated cells) and experimental groups (PGG-treated cells) were combined and clustered. There are three independent samples for each group. Each gene is represented by a single row of clustered boxes and each group is represented by a single column (left). Gene ontology classification based on biological processes and molecular functions (right).
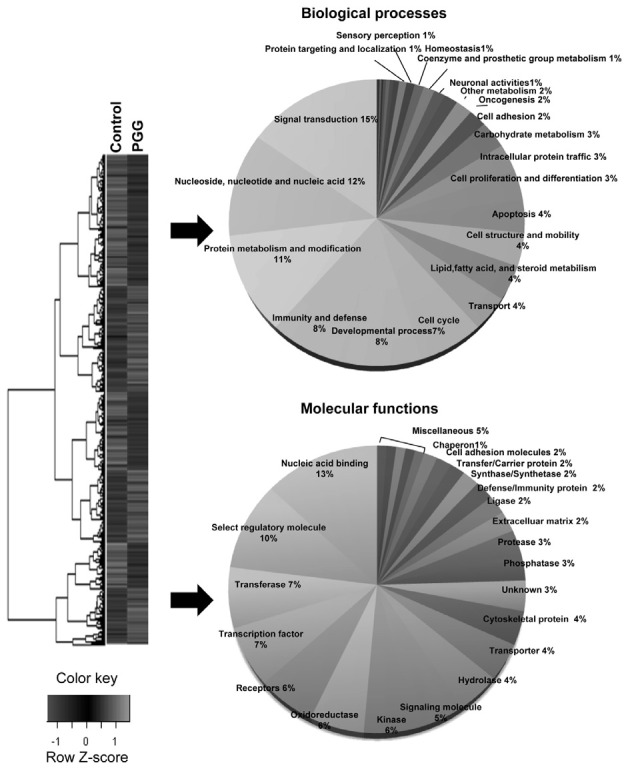
Table 3.
Down-regulation of genes based on comparison of gene expression between control (untreated) and experimental (PGG-treated) MDA MB-231 cells
| Genes | Accession no. | Symbol | Fold change* | p-value |
|---|---|---|---|---|
| Pyruvate metabolism | ||||
| Pyruvate carboxylase | NM_000920.2 | PC | -2.01 | 0.03 |
| Acyl-CoA synthetase short-chain family member 2 | NM_139274.1 | ACSS2 | -2.12 | 0.03 |
| Acetyl-Coenzyme A carboxylase alpha | NM_000664.3 | ACACA | -2.37 | 0.01 |
| Acylphosphatase 2, muscle type | NM_138448.2 | ACYP2 | -2.01 | 0.00 |
| Cell cycle | ||||
| Cyclin B1 | NM_031966.2 | CCNB1 | -3.47 | 0.00 |
| Cyclin B2 | NM_004701.2 | CCNB2 | -2.17 | 0.00 |
| Transforming growth factor, beta 2 | NM_003238.1 | TGFB2 | -3.00 | 0.00 |
| Ataxia telangiectasia mutated | NM_000051.3 | ATM | -2.39 | 0.02 |
| Glycolysis / Gluconeogenesis | ||||
| Acyl-CoA synthetase short-chain family member 2 | NM_139274.1 | ACSS2 | -2.12 | 0.03 |
| Aldehyde dehydrogenase 3 family, member B1 | NM_001030010.1 | ALDH3B1 | -2.95 | 0.00 |
| Fructose-1,6-bisphosphatase 1 | NM_000507.2 | FBP1 | -2.64 | 0.02 |
| Acylphosphatase 2, muscle type | NM_138448.2 | ACYP2 | -2.01 | 0.00 |
| TGF-beta signaling pathway | ||||
| Inhibitor of DNA binding 1 | NM_181353.1 | ID1 | -3.04 | 0.02 |
| Bone morphogenetic protein 4 | NM_001202.2 | BMP4 | -3.71 | 0.01 |
| E2F transcription factor 5, p130-binding | NM_001951.2 | E2F5 | -2.27 | 0.00 |
| Transforming growth factor, beta 2 | NM_003238.1 | TGFB2 | -3.00 | 0.00 |
| Tyrosine metabolism | ||||
| Protein arginine methyltransferase 2 | NM_206962.1 | PRMT2 | -2.03 | 0.01 |
| Aldehyde dehydrogenase 3 family, member B1 | NM_001030010.1 | ALDH3B1 | -2.95 | 0.00 |
| Catechol-O-methyltransferase | NM_000754.2 | COMT | -3.03 | 0.04 |
| MAPK signaling pathway | ||||
| Ribosomal protein S6 kinase, 90 kDa, polypeptide 2 | NM_021135.4 | RPS6KA2 | -2.14 | 0.02 |
| Arrestin, beta 1 | NM_004041.3 | ARRB1 | -2.30 | 0.00 |
| CD14 molecule | NM_000591.1 | CD14 | -5.54 | 0.00 |
| Protein phosphatase 3 (formerly 2B), catalytic subunit, beta isoform | NM_021132.1 | PPP3CB | -3.02 | 0.00 |
| Transforming growth factor, beta 2 | NM_003238.1 | TGFB2 | -3.00 | 0.00 |
| JAK/STAT signaling pathway | ||||
| Interferon (alpha, beta and omega) receptor 1 | NM_000629.2 | IFNAR1 | -2.62 | 0.03 |
| Sprouty homolog 1, antagonist of FGF signaling | NM_005841.1 | SPRY1 | -2.35 | 0.00 |
Validation of selected genes via real-time RT-PCR
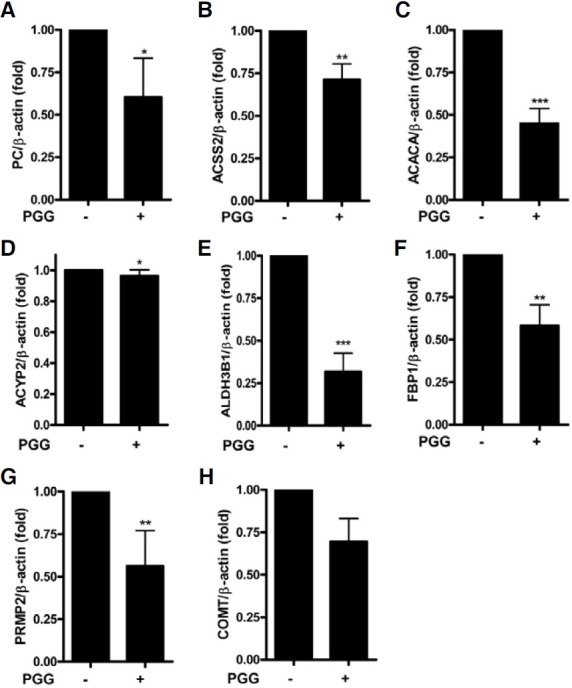
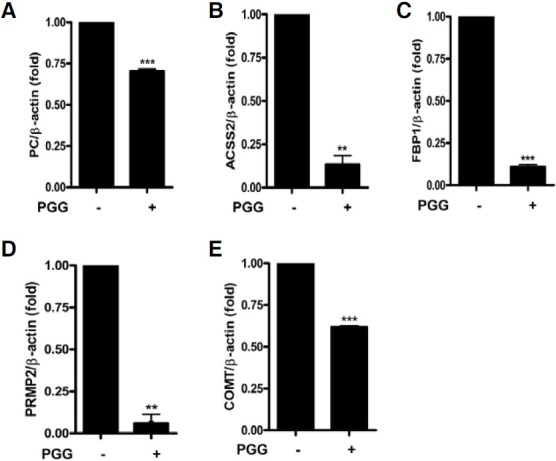
To prove the effects of PGG on cancer metabolism genes at mRNA level, real-time RT-PCR was conducted for 8 selected genes involved in pyruvate metabolism, glycolysis/gluconeogenesis and tyrosine metabolism. Consistent with the results of microarray analysis, PGG significantly suppressed the expression of PC, ACSS2, ACACA, ACYP2, ALDH3B1, FBP1, PRMT2 and COMT at mRNA levels in MDA-MB-231 cells (Figs. 4A-4H). The relative expression levels of each gene were normalized by dividing the expression of ACTB, a well known house keeping gene (Butte et al., 2001).
Fig. 4. Validation of gene expression by real-time RT-PCR in MDA-MB-231 cells. The results are normalized as a ratio of each specific mRNA signal to the β-actin (ACTB) gene signal within the same sample and the values expressed. (A) PC, (B) ACSS2, (C) ACACA, (D) ACYP2, (E) ALDH3B1, (F) FBP1, (G) PRMT2 and (H) COMT. Data were presented as means ± S.D. (n = 3). *p < 0.05, **p < 0.01 and ***p < 0.001 vs control.
To examine whether the inhibitory activity of PGG on cancer metabolism genes is reproducible in other breast cancer cell line, parallel assay was performed in MCF-7 cells treated with or without PGG. As shown in Fig. 5, PGG significantly reduced the mRNA expression of PC, ACSS2, FBP1, PRMT2 and COMT. In contrast, PGG had no significant effect on ACACA, ACYP2 and ALDH3B1 (data not shown).
Fig. 5. Validation of gene expression by realtime RT-PCR in MCF-7 cells. The results are normalized as a ratio of each specific mRNA signal to the β-actin (ACTB) gene signal within the same sample and the values expressed. (A) PC, (B) ACSS2, (C) FBP1, (D) PRMT2 and (E) COMT. Data were presented as means ± S.D. (n = 3). **p < 0.01 and ***p < 0.001 vs control.
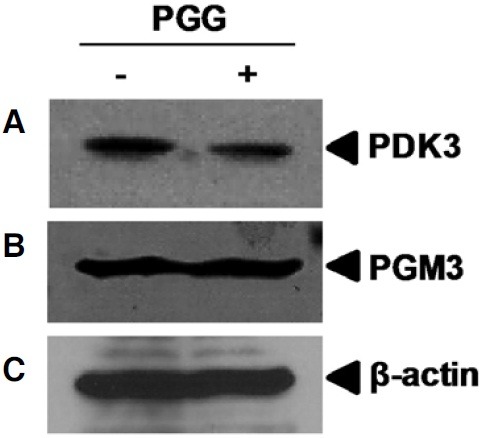
Effect of PGG on cancer metabolism-related protein expression
To test the possible relationship between anti-cancer activity of PGG and cancer metabolism, we performed Western blotting to determine the expression of proteins involving in the regulation of cancer metabolism and tumorigenesis (Lee et al., 2011a; Roche and Hiromasa, 2007). PGG suppressed the protein expression of pyruvate dehydrogenase 3 (PDK3), but not phosphoglucomutase 3 (PGM3).
DISCUSSION
Breast cancer is a lethal disease of breast tissues or ducts for women over the world. According to the National Cancer Institute (NCI) report, the incidence of breast cancer is more than 192,000 women each year in the United States. Treatment of breast cancer has been achieved by surgery, radiation and chemotherapy, or the combination despite significant side effects. Interestingly, recent studies have suggested natural products or compounds as potent anti-tumor agents to improve the therapeutic effect and reduce the side effects of breast cancer therapies (Madhusoodhanan et al., 2010; Ray et al., 2010; Reddish et al., 2010).
Fig. 6. Effect of PGG on cancer metabolism-related protein expression in MDA-MB-231 cells. Whole cell lysates were prepared and subjected to Western blotting to determine the expression of PDK3 (A) and PGM3 (B). β-actin (C) was used as an internal control.
PGG is one of plant secondary metabolites from many medicinal herbs such as Rhus chinensis Mill (Huh al., 2005), Acer truncatum Bunge (Zhang et al., 2008), Pelargonium inquinans Ait (Ji et al., 2005), and Paeonia lactiflora Pall (Lee et al., 2006). PGG has been reported to have anti-cancer activities via proapoptosis, anti-angiogenesis, anti-metastasis and anti-proliferation in many types of cancer including prostate cancer (Kuo et al., 2009), lung cancer (Huh et al., 2005), melanoma (Ho et al., 2002), liver cancer (Oh et al., 2001) and leukemia (Pan et al., 1999). PGG also showed anti-oxidative (Riedl and Hagerman, 2001), anti-mutagenic (Okuda et al., 1984), anti-inflammatory (Pan et al., 2000), anti-allergic (Cavalher-Machado et al., 2008) and anti-kidney stone formation (Lee et al., 2009a) activities in vitro and in vivo.
In this present study, we tried to elucidate the underlying biological effects in PGG-treated MDA-MB-231 breast cancer cells by microarray and real time RT-PCR. The genes regulated by PGG in MDA-MB-231 were classified using the KEGG database. Interestingly, we have detected the significant downregulation of genes related to cancer metabolism including pyruvate metabolism (PC, ACSS2, ACACA and ACYP2), glycolysis/gluconeogenesis (ACSS2, ALDH3B1, FBP1 and ACYP2) and tyrosine metabolism (PRMT2, ALDH3B1 and COMT) in PGG-treated MDA-MB-231 cells and confirmed the effect of PGG on cancer metabolism genes by real-time RT-PCR. In MCF-7 cells, PGG inhibited the mRNA expression of PC, ACSS2, FBP1, PRMT2 and COMT. In contrast, PGG had no significant effect on ACACA, ACYP2 and ALDH3B1 (data not shown). These results may be caused by the different characteristics between MDA-MB-231 (ER- estrogen-independent and mutant p53) and MCF-7 (ER+ estrogen-dependent and p53- wild-type) cells. However, further studies are required to confirm these biological mechanisms of PGG in vitro and in vivo in the future.
Pyruvate carboxylase (PC) is an enzyme to provide oxaloacetate (OAA) precursor for the citric acid cycle. Of interest, Rao and colleagues reported the cross-reactivity between multidrug resistance (MDR) and PC in cancer cells (Rao et al., 1994), suggesting that PC may be a possible target molecule for the treatment of drug resistant cancer cells. Acyl-CoA sythetase short-chain family member 2 (ACSS2) is a cytosolic enzyme that catalyzes the activation of acetate to acetyl-CoA for use in lipid synthesis and energy generation (Schwer et al., 2006). Recently, several studies reported the importance of ACSS2 for the radiolabeled acetate uptake and cell survival in tumor cells (Yoshii et al., 2009; Yun et al., 2009). Acetyl-CoA carboxylase (ACC) importantly functions to produce malonyl- CoA substrate for the biosynthesis of fatty acid by catalyzing the irreversible carboxylation of acetyl-CoA (Tong, 2005). The human genome includes the genes for two different ACCs ACC-alpha (ACACA) and ACC-beta (ACACB) (Brownsey et al., 1997). Many studies reported the potential role of ACC as a therapeutic target for cancer therapy (Beckers et al., 2007; Harwood et al., 2003; Jump et al., 2010). Acylphosphatase (ACYP) is an enzyme to hydrolyze the phosphoenzyme intermediate of different membrane pumps, particularly the Ca2+/ Mg2+-ATPase from sarcoplasmic reticulum of skeletal muscle and contains two isoenzymes (Calamai et al., 2005). ACYP overexpression was known to trigger SH-SY5Y neuroblastoma cell differentiation (Cecchi et al., 2004) and be associate with metastatic process in human colorectal cancer cells (Riley et al., 1997). Aldehyde dehydrogenase (ALDH) is an enzyme that catalyse the oxidation (dehydrogenation) of aldehydes and are metabolized by the body’s muscle and heart (Crabb et al., 2004). ALDH was highly activated in cyclophosphamideresistant L1210 leukemia cells (Hilton, 1984). Also, ALDH was down-regulated by retinoic acid in lung cancer cell lines, suggesting the capability of retinoids for lung cancer prevention and treatment (Moreb et al., 2005). Fructose-1,6-bisphosphatase 1 (FBP1) converts fructose-1,6-bisphosphate to fructose 6- phosphate in gluconeogenesis. Liu et al. (2010) recently suggested that promoter methylation of FBP1 can be used as a new biomarker for prognosis prediction of gastric cancer. It is not surprising that PGG suppressed the expression of protein arginine methyltransferase 2 (PRMT2) belonging to PRMT family proteins. Numerous studies have reported an essential function of PRMTs in oncogenesis and their potentials as therapeutic targets in human cancer (Cheung et al., 2007). Especially, PRMT2 was found to be capable of influencing estrogen receptor alpha (ERα) as a coactivator (Qi et al., 2002). Catechol- o-methyltransferase (COMT) is one of enzymes that degrade catecholamines and located in the postsynaptic neuron (Ulmanen et al., 1997). Many papers reported the association between COMT and breast cancer risk (Millikan et al., 1998; Onay et al., 2008; Wen et al., 2007; Xi et al., 2010).
In addition to cancer metabolism genes, the number of anticancer and signal transduction-related genes was up- or downregulated by PGG treatment. Anti-cancer activity of PGG is well described in our recent review (Zhang et al., 2009). Potential mechanisms responsible for anti-cancer activity of PGG include anti-angiogenesis (Huh et al., 2005; Lee et al., 2004), antiproliferation (Huh et al., 2005), pro-apoptosis (Chen and Lin, 2004; Pan et al., 1999) and anti-metastasis (Ho et al., 2002; Huh et al., 2005). PGG blocked tumor growth via inhibition of angiogenesis and stimulation of apoptosis through cyclooxygenase 2 (COX-2) and mitogen-activated protein kinase (MAPK)- dependent pathways in basic fibroblast growth factor (bFGF)- treated human umbilical vein endothelial cells (HUVECs) (Huh et al., 2005). PGG up-regulated heme oxygenase-1 expression by stimulating Nrf2 nuclear translocation in an extracellular signal- regulated kinase-dependent manner in HepG2 cells (Pae et al., 2006). Also, PGG inhibited signal transducer and activator of transcription 3 (STAT3) in prostate (Hu et al., 2008) and breast cancer cells (Lee et al., 2011b). Additionally, PGG suppressed the expression of vascular endothelial growth factor (VEGF) (Huh et al., 2005), aninhibited estrogen receptor alpha by lysosome- dependent depletion and modulated ErbB/PI3K/Akt pathway in human breast cancer MCF-7 cells (Hua et al., 2006). Furthermore, PGG inhibited phorbol myristate acetate (PMA)- induced interleukin-8 (IL-8) gene expression in human monocytic U937 cells through its inactivation of nuclear factorkappaB (NF-κB) (Oh et al., 2004). These previous results support our results of microarray analysis.
Glycolysis is a process by which glucose is turned into pyruvate and generally enhanced in cancer cells for ATP production (the Warburg effect) (Warburg et al., 1927). Under hypoxia and oncogenic mutations, high glucolytic rate promotes proliferation of cancer cells with the pyruvate to lactate conversion by activation of lactate dehydrogenase A and inactivation of pyruvate dehydrogenase (Feron, 2009). Tyrosine metabolic pathway is also highly active in tumors such as melanoma, small cell lung cancer and neuroectodermal tumors (Kobrinsky and Sjolander, 2006).
Anti-cancer drugs targeting cancer metabolism are associated with mitochondria dysfunction to induce apoptosis, reactive oxygen species (ROS) generation and so on (Pathania et al., 2009; Yu and Kim, 2011). Thus, cancer metabolism-related genes can be valuable targets to establish anti-cancer therapeutic strategies. To evaluate the relationship between PGG’s anti-cancer activity and cancer metabolism, the effect of PGG on several cancer metabolism-related molecules involving anticancer regulation was analyzed at protein levels. Several recent studies reported anti-cancer agents targeting pyruvate dehydrogenase complex/ pyruvate dehydrogenase kinase (PDH/ PDK) for reversal of Warburg effect (Warburg, 1956). Small molecules OSU-03012 (Lee et al., 2009b) and BAG156 (Weisberg et al., 2008) were also reported as PDK1 inhibitors to regulate anti-cancer activity. In our study, PGG decreased the level of PDK3, but not PDK1 (data not shown), in MDA-MB-231 cells. Furthermore, we examined whether or not PGG can influence phosphoglucomutase (PGM) expression. Benzene hexacarboxylic acid, 3-phos-phoglyceric acid (Scatena et al., 2008) and MJE3, a small molecule (Evans et al., 2005) were proposed as PGM inhibitors in cancer cells. We also recently reported that suppression of PGM3 is linked to sulforaphaneinduced apoptosis in prostate cancer cells (Lee et al., 2011a). However, PGG did not change PGM3 expression in MDA-MB- 231 cells. Our results imply that anti-cancer activity of PGG may be related to the down-regulation of PDK3. Further studies will be required to verify the relationship between anti-cancer activity and metabolism regulation corresponding to the identified gene expression by microarray in the near future.
Recent studies have suggested that the metabolic alteration of cancer cells is a promising therapeutic strategy (Pelicano et al., 2004; Xu et al., 2005). Thus, several agents such as 2- deoxyglucose (Geschwind et al., 2004; Maher et al., 2004), arsenic compounds (Geschwind et al., 2004) and 3-bromopyruvate (Geschwind et al., 2002) were known to regulate glycolysis by inhibiting ATP production. Also, nitisinone was suggested to block the tyrosine pathway as an effective drug for the treatment of neuroblastoma (Kobrinsky and Sjolander, 2006). Therefore, it is of importance to understand the effect of PGG on the metabolic property of MDA-MB-231 cells. Our study suggested the potential that PGG can be used as anticancer agent to modulate cancer metabolisms for breast cancer cells.
In summary, through genomic analysis, we demonstrate that PGG can mediate the expression of the cancer metabolism genes, the protein products of which are involved in pyruvate metabolism, glycolysis/gluconeogenesis and tyrosine metabolism as well as signal transduction in MDA-MB-231 cells. Realtime RT-PCR analysis confirmed the results from the microarray assay, in which cancer metabolism genes such as PC, ACSS2, ACACA, ACYP2, ALDH3B1, FBP1, PRMT2 and COMT were significantly downregulated at mRNA levels in PGGtreated MDA-MB-231 cells. Overall, these findings suggest the potential of PGG as a potent anticancer agent for treating breast cancer by targeting cancer metabolism genes. However, further studies are under way to determine anticancer mechanism of PGG via interfering with cancer metabolism pathway in this malignant disease in vitro and in vivo.
Acknowledgments
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (Ministry of Education, Science and Technology) (No. 2011-0063466).
References
- 1.Beckers A., Organe S., Timmermans L., Scheys K., Peeters A., Brusselmans K., Verhoeven G., Swinnen J.V. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. (2007);67:8180–8187. doi: 10.1158/0008-5472.CAN-07-0389. [DOI] [PubMed] [Google Scholar]
- 2.Brownsey R.W., Zhande R., Boone A.N. Isoforms of acetyl-CoA carboxylase: structures, regulatory properties and metabolic functions. Biochem. Soc. Trans. (1997);25:1232–1238. doi: 10.1042/bst0251232. [DOI] [PubMed] [Google Scholar]
- 3.Butte A.J., Dzau V.J., Glueck S.B. Further defining housekeeping, or “maintenance,” genes focus on “A compendium of gene expression in normal human tissues”. Physiol. Genomics. (2001);7:95–96. doi: 10.1152/physiolgenomics.2001.7.2.95. [DOI] [PubMed] [Google Scholar]
- 4.Calamai M., Canale C., Relini A., Stefani M., Chiti F., Dobson C.M. Reversal of protein aggregation provides evidence for multiple aggregated States. J. Mol. Biol. (2005);346:603–616. doi: 10.1016/j.jmb.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 5.Cavalher-Machado S.C., Rosas E.C., Brito Fde A., Heringe A.P., de Oliveira R.R., Kaplan M.A., Figueiredo M.R., Henriques M.G. The anti-allergic activity of the acetate fraction of Schinus terebinthifolius leaves in IgE induced mice paw edema and pleurisy. Int. Immunopharmacol. (2008);8:1552–1560. doi: 10.1016/j.intimp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Cecchi C., Liguri G., Fiorillo C., Bogani F., Gambassi M., Giannoni E., Cirri P., Baglioni S., Ramponi G. Acylphosphatase overexpression triggers SH-SY5Y differentiation towards neuronal phenotype. Cell. Mol. Life Sci. (2004);61:1775–1784. doi: 10.1007/s00018-004-4192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W.J., Lin J.K. Induction of G1 arrest and apoptosis in human jurkat T cells by pentagalloylglucose through inhibiting proteasome activity and elevating p27Kip1, p21Cip1/ WAF1, and Bax proteins. J. Biol. Chem. (2004);279:13496–13505. doi: 10.1074/jbc.M212390200. [DOI] [PubMed] [Google Scholar]
- 8.Cheung N., Chan L.C., Thompson A., Cleary M.L., So C.W. Protein arginine-methyltransferase-dependent oncogenesis. Nat. Cell Biol. (2007);9:1208–1215. doi: 10.1038/ncb1642. [DOI] [PubMed] [Google Scholar]
- 9.Crabb D.W., Matsumoto M., Chang D., You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc. Nutr. Soc. (2004);63:49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- 10.Evans M.J., Saghatelian A., Sorensen E.J., Cravatt B.F. Target discovery in small-molecule cell-based screens by in situ proteome reactivity profiling. Nat. Biotechnol. (2005);23:1303–1307. doi: 10.1038/nbt1149. [DOI] [PubMed] [Google Scholar]
- 11.Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. (2009);92:329–333. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Geschwind J.F., Ko Y.H., Torbenson M.S., Magee C., Pedersen P.L. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Res. (2002);62:3909–3913. [PubMed] [Google Scholar]
- 13.Geschwind J.F., Georgiades C.S., Ko Y.H., Pedersen P.L. Recently elucidated energy catabolism pathways provide opportunities for novel treatments in hepatocellular carcinoma. Expert Rev. Anticancer Ther. (2004);4:449–457. doi: 10.1586/14737140.4.3.449. [DOI] [PubMed] [Google Scholar]
- 14.Harwood H.J., Jr., Petras S.F., Shelly L.D., Zaccaro L.M., Perry D.A., Makowski M.R., Hargrove D.M., Martin K.A., Tracey W.R., Chapman J.G., et al. Isozyme-nonselective N-substituted bipiperidylcarboxamide acetyl-CoA carboxylase inhibitors reduce tissue malonyl-CoA concentrations, inhibit fatty acid synthesis, and increase fatty acid oxidation in cultured cells and in experimental animals. J. Biol. Chem. (2003);278:37099–37111. doi: 10.1074/jbc.M304481200. [DOI] [PubMed] [Google Scholar]
- 15.Hilton J. Role of aldehyde dehydrogenase in cyclophosphamide- resistant L1210 leukemia. Cancer Res. (1984);44:5156–5160. [PubMed] [Google Scholar]
- 16.Ho L.L., Chen W.J., Lin-Shiau S.Y., Lin J.K. Penta-Ogalloyl- beta-D-glucose inhibits the invasion of mouse melanoma by suppressing metalloproteinase-9 through down-regulation of activator protein-1. Eur. J. Pharmacol. (2002);453:149–158. doi: 10.1016/s0014-2999(02)02340-3. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann A.S., Gross G.G. Biosynthesis of gallotannins: formation of polygalloylglucoses by enzymatic acylation of 1,2,3,4,6-penta-O-galloylglucose. Arch. Biochem. Biophys. (1990);283:530–532. doi: 10.1016/0003-9861(90)90678-r. [DOI] [PubMed] [Google Scholar]
- 18.Hu H., Lee H.J., Jiang C., Zhang J., Wang L., Zhao Y., Xiang Q., Lee E.O., Kim S.H., Lu J. Penta-1,2,3,4,6-Ogalloyl- beta-D-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. (2008);7:2681–2691. doi: 10.1158/1535-7163.MCT-08-0456. [DOI] [PubMed] [Google Scholar]
- 19.Hu H., Chai Y., Wang L., Zhang J., Lee H.J., Kim S.H., Lu J. Pentagalloylglucose induces autophagy and caspaseindependent programmed deaths in human PC-3 and mouse TRAMP-C2 prostate cancer cells. Mol. Cancer Ther. (2009a);8:2833–2843. doi: 10.1158/1535-7163.MCT-09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu H., Zhang J., Lee H.J., Kim S.H., Lu J. Penta- O-galloyl-beta-D-glucose induces S- and G(1)-cell cycle arrests in prostate cancer cells targeting DNA replication and cyclin D1. Carcinogenesis. (2009b);30:818–823. doi: 10.1093/carcin/bgp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hua K.T., Way T.D., Lin J.K. Pentagalloylglucose inhibits estrogen receptor alpha by lysosome-dependent depletion and modulates ErbB/PI3K/Akt pathway in human breast cancer MCF-7 cells. Mol. Carcinog. (2006);45:551–560. doi: 10.1002/mc.20226. [DOI] [PubMed] [Google Scholar]
- 22.Huh J.E., Lee E.O., Kim M.S., Kang K.S., Kim C.H., Cha B.C., Surh Y.J., Kim S.H. Penta-O-galloyl-beta-D-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: roles of cyclooxygenase-2 and mitogen- activated protein kinase pathways. Carcinogenesis. (2005);26:1436–1445. doi: 10.1093/carcin/bgi097. [DOI] [PubMed] [Google Scholar]
- 23.Ji M.S., Piao X.L., Jin Y.L., Park R.D. Anticoagulant 1,2,3,4,6-pentagalloyl-beta-D-glucopyranose isolated from geranium (Pelargonium inquinans Ait). Arch. Pharm. Res. (2005);28:1037–1041. doi: 10.1007/BF02977398. [DOI] [PubMed] [Google Scholar]
- 24.Jump D.B., Torres-Gonzalez M., Olson L.K. Soraphen A, an inhibitor of acetyl CoA carboxylase activity, interferes with fatty acid elongation. Biochem. Pharmacol. (2010);81:649–660. doi: 10.1016/j.bcp.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobrinsky N.L., Sjolander D.E. Response of metastatic recurrent neuroblastoma to nitisinone: a modulator of tyrosine metabolism. Pediatr. Blood Cancer. (2006);46:517–520. doi: 10.1002/pbc.20420. [DOI] [PubMed] [Google Scholar]
- 26.Kuo P.T., Lin T.P., Liu L.C., Huang C.H., Lin J.K., Kao J.Y., Way T.D. Penta-O-galloyl-beta-D-glucose suppresses prostate cancer bone metastasis by transcriptionally repressing EGF-induced MMP-9 expression. J. Agric. Food Chem. (2009);57:3331–3339. doi: 10.1021/jf803725h. [DOI] [PubMed] [Google Scholar]
- 27.Lee S.J., Lee H.M., Ji S.T., Lee S.R., Mar W., Gho Y.S. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose blocks endothelial cell growth and tube formation through inhibition of VEGF binding to VEGF receptor. Cancer Lett. (2004);208:89–94. doi: 10.1016/j.canlet.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Lee S.J., Lee H.K., Jung M.K., Mar W. In vitro antiviral activity of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose against he-patitis B virus. Biol. Pharm. Bull. (2006);29:2131–2134. doi: 10.1248/bpb.29.2131. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.H., Yehl M., Ahn K.S., Kim S.H., Lieske J.C. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose attenuates renal cell migration, hyaluronan expression, and crystal adhesion. Eur. J. Pharmacol. (2009a);606:32–37. doi: 10.1016/j.ejphar.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Lee T.X., Packer M.D., Huang J., Akhmametyeva E.M., Kulp S.K., Chen C.S., Giovannini M., Jacob A., Welling D.B., Chang L.S. Growth inhibitory and anti-tumour activities of OSU-03012, a novel PDK-1 inhibitor, on vestibular schwannoma and malignant schwannoma cells. Eur. J. Cancer. (2009b);45:1709–1720. doi: 10.1016/j.ejca.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C.H., Jeong S.J., Yun S.M., Kim J.H., Lee H.J., Ahn K.S., Won S.H., Kim H.S., Zhu S., Chen C.Y., et al. Downregulation of phosphoglucomutase 3 mediates sulforaphaneinduced cell death in LNCaP prostate cancer cells. Proteome Sci. (2011a);8:67. doi: 10.1186/1477-5956-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H.J., Seo N.J., Jeong S.J., Park Y., Jung D.B., Koh W., Lee E.O., Ahn K.S., Lu J., Kim S.H. Oral administration of Penta-O-galloyl-{beta}-D-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with JAK1-STAT3 Inhibition. Carcinogenesis. (2011b) doi: 10.1093/carcin/bgr015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y., Kim J., Li J., Liu F., Liu X., Himmeldirk K., Ren Y., Wagner T.E., Chen X. Natural anti-diabetic compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway. Biochem. Biophys. Res. Commun. (2005);336:430–437. doi: 10.1016/j.bbrc.2005.08.103. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Wang X., Zhang J., Lam E.K., Shin V.Y., Cheng A.S., Yu J., Chan F.K., Sung J.J., Jin H.C. Warburg effect revisited: an epigenetic link between glycolysis and gastric carcinogenesis. Oncogene. (2010);29:442–450. doi: 10.1038/onc.2009.332. [DOI] [PubMed] [Google Scholar]
- 35.Madhusoodhanan R., Natarajan M., Singh J.V., Jamgade A., Awasthi V., Anant S., Herman T.S., Aravindan N. Effect of black raspberry extract in inhibiting NFkappa B dependent radioprotection in human breast cancer cells. Nutr. Cancer. (2010);62:93–104. doi: 10.1080/01635580903191494. [DOI] [PubMed] [Google Scholar]
- 36.Maher J.C., Krishan A., Lampidis T.J. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother. Pharmacol. (2004);53:116–122. doi: 10.1007/s00280-003-0724-7. [DOI] [PubMed] [Google Scholar]
- 37.Millikan R.C., Pittman G.S., Tse C.K., Duell E., Newman B., Savitz D., Moorman P.G., Boissy R.J., Bell D.A. Catechol-O-methyltransferase and breast cancer risk. Carcinogenesis. (1998);19:1943–1947. doi: 10.1093/carcin/19.11.1943. [DOI] [PubMed] [Google Scholar]
- 38.Moreb J.S., Gabr A., Vartikar G.R., Gowda S., Zucali J.R., Mohuczy D. Retinoic acid down-regulates aldehyde dehydrogenase and increases cytotoxicity of 4-hydroperoxycyclophosphamide and acetaldehyde. J. Pharmacol. Exp. Ther. (2005);312:339–345. doi: 10.1124/jpet.104.072496. [DOI] [PubMed] [Google Scholar]
- 39.Oh G.S., Pae H.O., Oh H., Hong S.G., Kim I.K., Chai K.Y., Yun Y.G., Kwon T.O., Chung H.T. In vitro anti-proliferative effect of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose on human hepatocellular carcinoma cell line, SK-HEP-1 cells. Cancer Lett. (2001);174:17–24. doi: 10.1016/s0304-3835(01)00680-2. [DOI] [PubMed] [Google Scholar]
- 40.Oh G.S., Pae H.O., Choi B.M., Lee H.S., Kim I.K., Yun Y.G., Kim J.D., Chung H.T. Penta-O-galloyl-beta-D-glucose inhibits phorbol myristate acetate-induced interleukin-8 [correction of intereukin-8] gene expression in human monocytic U937 cells through its inactivation of nuclear factor-kappaB. Int. Immunopharmacol. (2004);4:377–386. doi: 10.1016/j.intimp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Okuda T., Mori K., Hayatsu H. Inhibitory effect of tannins on direct-acting mutagens. Chem. Pharm. Bull. (Tokyo) (1984);32:3755–3758. doi: 10.1248/cpb.32.3755. [DOI] [PubMed] [Google Scholar]
- 42.Onay U.V., Aaltonen K., Briollais L., Knight J.A., Pabalan N., Kilpivaara O., Andrulis I.L., Blomqvist C., Nevanlinna H., Ozcelik H. Combined effect of CCND1 and COMT polymorphisms and increased breast cancer risk. BMC Cancer. (2008);8:6. doi: 10.1186/1471-2407-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pae H.O., Oh G.S., Jeong S.O., Jeong G.S., Lee B.S., Choi B.M., Lee H.S., Chung H.T. 1,2,3,4,6-penta-Ogalloyl- beta-D-glucose up-regulates heme oxygenase-1 expression by stimulating Nrf2 nuclear translocation in an extracellular signal-regulated kinase-dependent manner in HepG2 cells. World J. Gastroenterol. (2006);12:214–221. doi: 10.3748/wjg.v12.i2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan M.H., Lin J.H., Lin-Shiau S.Y., Lin J.K. Induction of apoptosis by penta-O-galloyl-beta-D-glucose through activation of caspase-3 in human leukemia HL-60 cells. Eur. J. Pharmacol. (1999);381:171–183. doi: 10.1016/s0014-2999(99)00549-x. [DOI] [PubMed] [Google Scholar]
- 45.Pan M.H., Lin-Shiau S.Y., Ho C.T., Lin J.H., Lin J.K. Suppression of lipopolysaccharide-induced nuclear factorkappaB activity by theaflavin-3,3′-digallate from black tea and other polyphenols through down-regulation of IkappaB kinase activity in macrophages. Biochem. Pharmacol. (2000);59:357–367. doi: 10.1016/s0006-2952(99)00335-4. [DOI] [PubMed] [Google Scholar]
- 46.Pathania D., Millard M., Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv. Drug Deliv. Rev. (2009);61:1250–1275. doi: 10.1016/j.addr.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Pelicano H., Carney D., Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat. (2004);7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Qi C., Chang J., Zhu Y., Yeldandi A.V., Rao S.M., Zhu Y.J. Identification of protein arginine methyltransferase 2 as a coactivator for estrogen receptor alpha. J. Biol. Chem. (2002);277:28624–28630. doi: 10.1074/jbc.M201053200. [DOI] [PubMed] [Google Scholar]
- 49.Rao V.V., Anthony D.C., Piwnica-Worms D. MDR1 gene-specific monoclonal antibody C494 cross-reacts with pyruvate carboxylase. Cancer Res. (1994);54:1536–1541. [PubMed] [Google Scholar]
- 50.Ray R.B., Raychoudhuri A., Steele R., Nerurkar P. Bitter melon (Momordica charantia) extract inhibits breast cancer cell proliferation by modulating cell cycle regulatory genes and promotes apoptosis. Cancer Res. (2010);70:1925–1931. doi: 10.1158/0008-5472.CAN-09-3438. [DOI] [PubMed] [Google Scholar]
- 51.Reddish J.M., Ye W., Lin Y.C., Wick M. (-)-Gossypol containing hen sera and a myosin (-)-gossypol conjugate reduces the proliferation of MCF-7 cells. Anti Cancer Res. (2010);30:439–444. [PubMed] [Google Scholar]
- 52.Riedl K.M., Hagerman A.E. Tannin-protein complexes as radical scavengers and radical sinks. J. Agric. Food Chem. (2001);49:4917–4923. doi: 10.1021/jf010683h. [DOI] [PubMed] [Google Scholar]
- 53.Riley H.D., Macnab J., Farrell T.J., Cohn K. The expression of acylphosphatase is associated with the metastatic phenotype in human colorectal tumors. Carcinogenesis. (1997);18:2453–2455. doi: 10.1093/carcin/18.12.2453. [DOI] [PubMed] [Google Scholar]
- 54.Roche T.E., Hiromasa Y. Pyruvate dehydrogenase kinase regulatory mechanisms and inhibition in treating diabetes, heart ischemia, and cancer. Cell. Mol. Life Sci. (2007);64:830–849. doi: 10.1007/s00018-007-6380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scatena R., Bottoni P., Pontoglio A., Mastrototaro L., Giardina B. Glycolytic enzyme inhibitors in cancer treatment. Expert Opin. Investig. Drugs. (2008);17:1533–1545. doi: 10.1517/13543784.17.10.1533. [DOI] [PubMed] [Google Scholar]
- 56.Schwer B., Bunkenborg J., Verdin R.O., Andersen J.S., Verdin E. Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc. Natl. Acad. Sci. USA. (2006);103:10224–10229. doi: 10.1073/pnas.0603968103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong L. Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. (2005);62:1784–1803. doi: 10.1007/s00018-005-5121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ulmanen I., Peranen J., Tenhunen J., Tilgmann C., Karhunen T., Panula P., Bernasconi L., Aubry J.P., Lundstrom K. Expression and intracellular localization of catechol Omethyltransferase in transfected mammalian cells. Eur. J. Biochem. (1997);243:452–459. doi: 10.1111/j.1432-1033.1997.0452a.x. [DOI] [PubMed] [Google Scholar]
- 59.Warburg O. On the origin of cancer cells. Science. (1956);123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 60.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. (1927);8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weisberg E., Banerji L., Wright R.D., Barrett R., Ray A., Moreno D., Catley L., Jiang J., Hall-Meyers E., Sauveur-Michel M., et al. Potentiation of antileukemic therapies by the dual PI3K/PDK-1 inhibitor, BAG956: effects on BCR-ABL- and mutant FLT3-expressing cells. Blood. (2008);111:3723–3734. doi: 10.1182/blood-2007-09-114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen W., Ren Z., Shu X.O., Cai Q., Ye C., Gao Y.T., Zheng W. Expression of cytochrome P450 1B1 and catechol-Omethyltransferase in breast tissue and their associations with breast cancer risk. Cancer Epidemiol. Biomarkers Prev. (2007);16:917–920. doi: 10.1158/1055-9965.EPI-06-1032. [DOI] [PubMed] [Google Scholar]
- 63.Xi B., Zeng T., Liu W. Catechol-O-methyltransferase Val158Met polymorphism in breast cancer risk. Breast Cancer Res. Treat. (2010);126:839–841. doi: 10.1007/s10549-010-1337-6. [DOI] [PubMed] [Google Scholar]
- 64.Xu R.H., Pelicano H., Zhou Y., Carew J.S., Feng L., Bhalla K.N., Keating M.J., Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. (2005);65:613–621. [PubMed] [Google Scholar]
- 65.Yoshii Y., Waki A., Furukawa T., Kiyono Y., Mori T., Yoshii H., Kudo T., Okazawa H., Welch M.J., Fujibayashi Y. Tumor uptake of radiolabeled acetate reflects the expression of cytosolic acetyl-CoA synthetase: implications for the mechanism of acetate PET. Nucl. Med. Biol. (2009);36:771–777. doi: 10.1016/j.nucmedbio.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 66.Yu J.S., Kim A.K. Wogonin induces apoptosis by activation of ERK and p38 MAPKs signaling pathways and generation of reactive oxygen species in human breast cancer cells. Mol. Cells. (2011);31:327–335. doi: 10.1007/s10059-011-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yun M., Bang S.H., Kim J.W., Park J.Y., Kim K.S., Lee J.D. The importance of acetyl coenzyme A synthetase for 11C-acetate uptake and cell survival in hepatocellular carcinoma. J. Nucl. Med. (2009);50:1222–1228. doi: 10.2967/jnumed.109.062703. [DOI] [PubMed] [Google Scholar]
- 68.Zhang F., Luo S.Y., Ye Y.B., Zhao W.H., Sun X.G., Wang Z.Q., Li R., Sun Y.H., Tian W.X., Zhang Y.X. The antibacterial efficacy of an aceraceous plant [Shantung maple (Acer truncatum Bunge)] may be related to inhibition of bacterial betaoxoacyl- acyl carrier protein reductase (FabG). Biotechnol. Appl. Biochem. (2008);51:73–78. doi: 10.1042/BA20070255. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J., Li L., Kim S.H., Hagerman A.E., Lu J. Anticancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. (2009);26:2066–2080. doi: 10.1007/s11095-009-9932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


