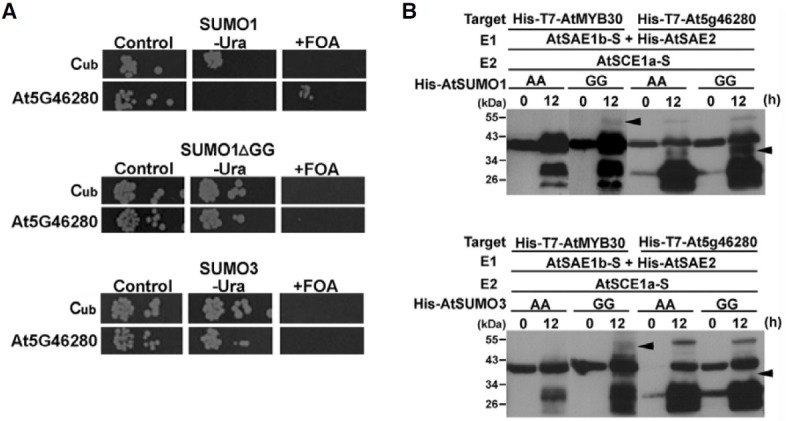
Fig. 5. Specific interaction and sumoylation pattern between SUMO1 and MCM3 (At5G46280) protein. (A) AtSUMO1 specifically interacts with MCM3 (At5G46280) protein in the yeast split ubiquitin system. Shown are interactions with MCM3 (At5G46280) protein and SUMOs (SUMO1, SUMO1ΔGG, or SUMO3) by serial dilutions of cells co-expressing Cub or Cub-SUMOs (SUMO1, SUMO1ΔGG, and SUMO3) fusions, respectively, on plates lacking tryptophan and histidine (control) additionally lacking uracil (-Ura) or containing 5-FOA (+FOA). Proteins were expressed from single copy vectors. (B) Specific sumoylation pattern of MCM3 (At5G46280) protein by AtSUMO1 using AtSAE1b as a subunit of the E1 heterodimer. Escherichia coli BL21(DE3) was transformed with each construct. Transformed E. coli cells were incubated at 37℃ until the OD600 reached 1.0, and the expression of tagged proteins and sumoylation was then induced overnight with 0.4 mM IPTG at 25℃. Cell lysates were prepared and Western blotting was carried out using an anti-T7-tag antibody. Sumoylated MCM3 (At5G46280) protein was observed as bands shifted to higher molecular weight in lanes using mature AtSUMO (GG) (indicated as GG), but not in lanes using mutated AtSUMO (AA) (indicated as AA). AtMYB30 was used as positive control for the sumoylation assay in an E. coli system. Arrow heads indicate sumoylated proteins.