Abstract
Xenopus laevis oocytes are commonly used to study the biophysical and pharmacological properties of foreign ion channels and receptors, but little is known about those endogenously expressed in their enveloping layer of follicular cells (FCs). Whole-cell recordings and the perforated patch-clamp technique in cultured FCs held at -60 mV revealed that ATP (20-250 μM) generates inward currents of 465 ± 93 pA (mean ± standard error) in ∼60% of the FCs studied, whereas outward currents of 317 ± 100 pA were found in ∼5% of the cells. The net effect of ATP on the FCs was to activate both mono- and biphasic inward currents, with an associated increase in membrane chloride conductance. Two-microelectrode voltage-clamp recordings of nude oocytes held at -60 mV disclosed that ATP elicited biphasic inward currents, corresponding to the well-known Fin and Sin-like currents. ATP receptor antagonists like suramin, TNP-ATP, and RB2 did not inhibit any of these responses. On the other hand, when using wholecell recordings, 1 μM Ang II yielded smooth inward currents of 157 ± 45 pA in ∼16% of the FC held at -60 mV. The net Ang II response, mediated by the activation of the AT1 receptor, was a chloride current inhibited by 10 nM ZD7155. This study will help to better understand the roles of ATP and Ang II receptors in the physiology of X. laevis oocytes.
Keywords: chloride current, follicular cells, oocytes, transmitters, whole-cell recording
INTRODUCTION
Oocytes from the African frog (Xenopus laevis) have been widely used to study diverse biological processes associated with ion channels and membrane receptors (Miledi et al., 1989). The electrophysiological responses generated by follicular cells (FCs) are scarce and poorly understood, given that most of the relevant information has been obtained indirectly from follicles (individual oocytes each surrounded by a monolayer of FCs). For example, diverse substances such as acetylcholine, adenosine, noradrenaline, serotonin, and gonadotrophic hormones have been tested for their effect on follicles, primarily to understand their roles in oocyte maturation and their metabolism as second messengers (Arellano et al., 1996; Miledi, 1982).
The plasma membranes of FCs and oocytes are in continuous communication through gap junctions. Some hormones and transmitters induce ion currents in FCs that affect both the oocyte and the ooplasm, generating important signals and second messengers that transmit information between these cells (Honoré and Lazdunski, 1993; Miledi and Woodward, 1989; Sandberg et al., 1990). It is known that X. oocytes express receptors for angiotensin II (Ang II) and ATP. In these oocytes, Ang II receptors activate a calcium-dependent chloride current through the IP3/calcium-release pathway (Matute et al., 1994; Miledi, 1982; Sandberg et al., 1990). Further investigations showed that after defolliculation (the process of removing FCs from the oocyte membrane, see ref. Miledi and Woodward, 1989), the Ang II responses still prevailed (Arellano et al., 1996). These results suggested the presence of Ang II receptors in the oocyte membrane. Nonetheless, the FCs had not been tested in isolation. On the other hand, ATP induces at least two types of chloride inward currents in follicles, a fast desensitizing current (Fin) and a slow current (Sin), both induced by the intracellular calcium metabolism (Arellano et al., 1996). However, the ATP responses observed in follicles are absent after defolliculation (Arellano et al., 1996; King et al., 1996; Miledi and Woodward, 1989). This finding suggested that ATP receptors were expressed specifically in the FC membranes, but the response of individual FCs to ATP has not yet been directly investigated. Clearly, these examples illustrate the intrinsic communication between the FCs and the oocyte membrane. However, the participation of the Ang II and ATP receptors in generating particular ion-currents in isolated FCs has not been studied and is important to decipher the role of these receptors and better understand the physiology of the oocyte-FC interaction. Thus, this study was designed to determine the electrical characteristics of the responses produced by ATP and Ang II in FCs maintained in culture.
MATERIALS AND METHODS
Follicular cell isolation and culture
All experimental procedures were in compliance with the ethical policies of animal care and handling of the National University (UNAM). X. laevis oocytes at stages V and VI were selected to isolate the FCs. The epithelium and theca were removed, using fine forceps, under a binocular-dissecting stereoscope to obtain ‘unzipped oocytes’ (Miledi and Woodward, 1989). This procedure left exposed the monolayer of FCs enveloping the vitelline membrane of the oocyte. At least 150 ‘unzipped oocytes’ were incubated in a Ca2+-free Ringer’s solution (see Electrophysiology) for 5 min to facilitate cell segregation, followed by defolliculation during a 30-min incubation with 0.25 mg/ml collagenase type I (Sigma). The oocytes were then rinsed with sterile modified Barth’s solution (see Electrophysiology), placed in a sigmacote- treated vial (Sigma) to avoid FC adhesion, and gently spun (30 rpm) for up to 2 h in modified Barth’s medium supplemented with 1% fetal bovine serum (FBS). The dislodged FCs were recovered with a polished Pasteur pipette, plated on a poly-L-ornithine-treated glass coverslip, and allowed to settle overnight on the substrate. The Barth’s medium was replaced by 80% L-15 culture medium (supplemented with 5% FBS). The FCs were maintained in culture (up to 14 days) at 18℃ for subsequent electrophysiological recordings.
Electrophysiology in cultured follicular cells
In this study, primary cultures of FC were first identified by morphology under an inverted light microscope (Olympus IX51). The FC had the same morphological characteristics and dimensions as described by Miledi and Woodward (1989). Isolated cells were placed in the perfusion chamber and tested with different concentrations of ATP or with 1 μM Ang II to investigate if any effect was induced by these chemical transmitters.
Membrane current responses activated by ATP or Ang II were recorded from cultured FC using the whole-cell voltageclamp technique (Hamill et al., 1981). The experiments were performed at room temperature (20-23℃) between days 2 and 14 of culture and in a bath continuously perfused with frog Ringer’s solution (in mM): NaCl 115, KCl 2, CaCl2 1.8, HEPES 5, adjusted to pH 7.0 (Miledi and Woodward, 1989). For the Ca2+-free Ringer solution, Ca2+ was not added to the Ringer solution and was replaced with Na+ to maintain osmolarity. The patch pipettes were made from borosilicate glass capillaries and filled with an intracellular solution containing (in mM): KCl 115, NaCl 2, EGTA 5, HEPES 5 (pH 7.0), giving a resistance of 3-5 MΩ. For the perforate-patch clamp recordings (Marty and Neher, 1995; Neher and Sakmann, 1995), the pipettes were filled with a solution containing (in mM): KCl 115, NaCl 2, EGTA 5, HEPES 5, and 100 μg/ml nystatin (adjusted to pH 7.0). The tip was filled with nystatin-free intracellular solution giving a resistance of 3-5 MΩ. To investigate the ion selectivity of the channel activated by the effects of ATP or Ang II on the FCs, current to voltage (I-V) relationships were constructed by changing the holding potential according to the following protocol: 200-ms voltage pulses from -120 to 80 mV in 20-mV increments or 1-s voltage ramps from -120 to 80 mV. Transient capacitance and leak currents were digitally substracted. The currents were amplified, filtered at 5 kHz, and digitalized at 100 Hz (for transmitter-induced currents), 3.3 kHz (for voltage pulses), and 0.5 kHz (for voltage ramps). The data were analyzed with the pClamp 8.2 software (Axon Instruments, USA). For the experiments in which the chloride equilibrium potential was changed to -40 mV, the intracellular solution in the patch pipettes contained (in mM): NaCl 2, KCl 78, K-Gluconate 37, HEPES 5, and EGTA 10, adjusted to pH 7.0. In the experiments in which chloride was substituted by gluconate, the intracellular solution in the patch pipettes contained (in mM): Nagluconate 2, K-gluconate 115, HEPES 5, and EGTA 5, adjusted to pH 7.0, and the frog Ringer’s solution contained (in mM): Na-gluconate 115, K-gluconate 2, Ca-gluconate 1.8, and HEPES 5, adjusted to pH 7.0.
Electrophysiology in oocytes
Xenopus oocytes at stages V and VI were manually isolated from the ovaries and maintained at 16-18℃ in Barth’s solution containing (in mM): 88 NaCl, 1 KCl, 0.33 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4, 2.4 NaHCO3, 5 HEPES (pH 7.4), and 0.1 mg/ml gentamicin sulfate. ATP or Ang II responses were obtained, immediately after removing the connective tissues and the FC monolayer from follicles, using the two-microelectrode voltageclamp technique (Miledi, 1982). The oocytes were placed in a perfusion chamber (volume ~0.1 ml), and ATP or Ang II was continuously perfused in Ringer solution (see above) at room temperature (20-23℃) at rates of 7-10 ml/min. Membrane currents elicited by Ang II or ATP were recorded with a commercial amplifier (Warner OC-752A; Warner Instruments Corp., USA), monitored with an oscilloscope (Nicolet 310; Nicolet Instruments Corp, USA), and stored for subsequent analyses. A 1-s voltage-ramp (from -120 to 40 mV) was applied to the cells from a holding potential of -60 mV. Data are expressed as the mean ± standard error (SE).
Drugs
Ang II (1 μM) was prepared daily from a frozen Ang II stock solution (Sigma-RBI). The AT1 receptor antagonist ZD7155 (Tocris, Cookson Inc.) has an IC50 of 3.8 nM in guinea-pig adrenal gland (Oldham et al., 1993), thus this antagonist was prepared daily to a final concentration of 15 nM from a frozen stock solution. ATP solutions (20-250 μM) were made daily from adenosine 5′-triphosphate disodium salt (Sigma-RBI). Solutions of 3-100 μM suramin, 10 μM 2′, 3′-O-(2, 4, 6-trinitrophenyl)- adenosine 5′-triphosphate monolithium trisodium salt (TNP-ATP), and 10 μM Reactive blue 2 (RB-2) were also prepared daily. Suramin, TNP-ATP, and RB2 (Sigma - RBI) are non-selective purinergic antagonists with average IC50 values of 5, 10, and 6 μM, respectively. All three substances were diluted in Ringer’s solution and applied externally to the cells by switching the perfusion line.
RESULTS
ATP responses in follicular cells
Isolated follicular cells held at -60 mV were tested with ATP at different concentrations (20-250 μM) yielding inward currents of 465 ± 93 pA in 54 of 91 FCs and outward currents of 317 ± 100 pA in 5 of 91. ATP, independently of the concentration, induced two types of inward currents: 1) a fast inward (Fin) current that decays promptly in the continuous presence of ATP and that does not return to the base line until after ATP is removed from the perfusion bath (n = 30); and 2) a Fin-current followed by a second inward current that exhibits a slower decay rate (n = 9).
Figure 1 shows sample recordings of the two types of currents generated by ATP in the FCs. Figure 1A shows a sample recording of the most common type of current found; this current developed promptly upon exposure to ATP and shows two decay components, but it does not seem to desensitize even after long exposures to ATP and does not return the base line until the agonist is removed. The second type of ATP-current exhibited biphasic inward currents. These Fin-currents (442 ± 136 pA; n = 17) presented oscillations, and the Sin-currents (660 ± 168 pA; n = 9) decayed even in the presence of ATP (Fig. 1C).
Fig. 1. Ion currents induced by ATP and Ang II in isolated FCs and oocytes. The time of application (up arrows) and removal (down arrows) of the indicated substances is shown. (A), (C), and (E) are sample responses obtained in 17, 9, and 10 FCs, respectively. (B), (D), and (F) are sample responses generated in 6, 3, and 4 oocytes, respectively. The oocytes, from two frogs, were recorded within 1 h after their extraction.
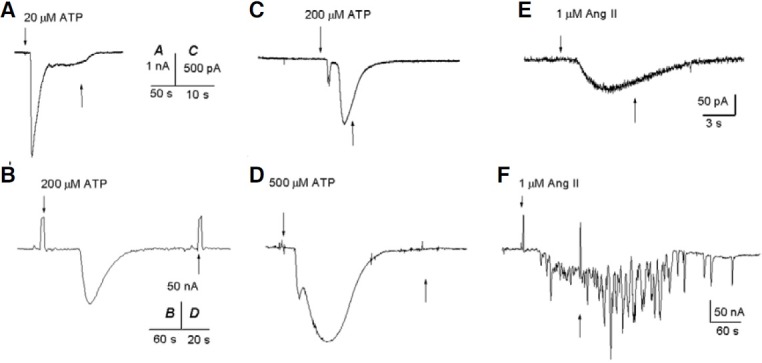
This current is similar to that activated by ATP in epitheliumremoved follicles, and it is assumed to originate in the FCs (Arellano et al., 1996). However, as shown in Fig. 1B, ATP induced inward currents in oocytes with characteristics similar to those described above for FCs. This current reached 125 nA and decayed in the presence of ATP, returning to base line after ∼70 s. These inward currents were similar to those found in a few oocytes that responded to ATP (Arellano et al., 1996), probably due to FCs that remained attached to the oocytes.
In follicles, the Fin- and Sin-currents are activated by ATP, but they disappear after defolliculation (Arellano et al., 1996). Thus, we tested ATP in seven nude oocytes from two frogs in which ATP had generated these currents in isolated cells (Fig. 1D). It was interesting to find that the Sin-currents decayed in the presence of ATP in both the FCs and the oocytes, suggesting in fact that some FCs remained attached to the oocytes.
In addition, we tested the effect of three different ATP receptor antagonists on the follicular cells. ATP responses in these cells were not blocked by 100 μM suramin, 10 μM TNP-ATP, or 10 μM RB-2 (in at least 3 experiments for each antagonist; data not shown), suggesting the presence of an unusual ATP receptor or a peculiar assembly of receptor subunits.
Because the ATP-inward currents were more abundant in the FCs, we decided to determine some of their electrical characteristics and the ion responsible for their generation. Hence, voltage-ramps were applied before, during, and after the response activated by applying ATP on the FCs. Analysis of the current to voltage (I-V) relationships revealed an increase in the membrane conductance in 75% of the cells; however, in the remaining 25% it was decreased (Fig. 2B).
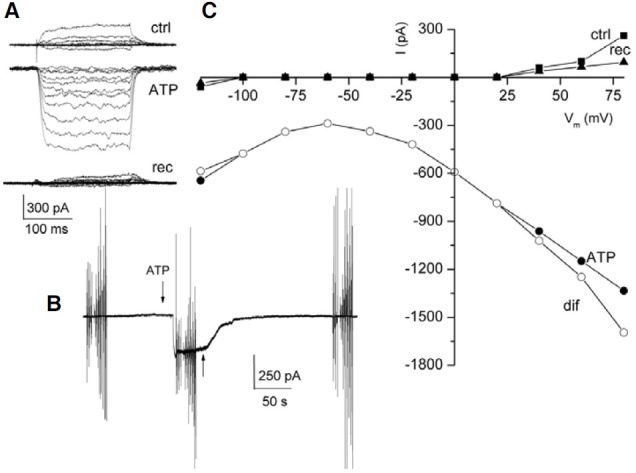
Fig. 2. Typical ATP responses found in the FCs and oocytes. (A) and (C) show sample ATP-currents from FCs and oocytes, respectively; the arrows indicate the application of 200 (A) and 250 μM ATP (C). The vertical lines show membrane currents activated by voltage ramp protocols. (B) and (D) are the I-V relationships generated during the peak of the inward current (from A) and the Fin- and outward current (from C), respectively. Ctrl, rec, are control and recovery ramps; ATP indicates the voltage-ramp applied during the ATP-current; dif represents the net ATP-current induced. All cells were held at -60 mV.
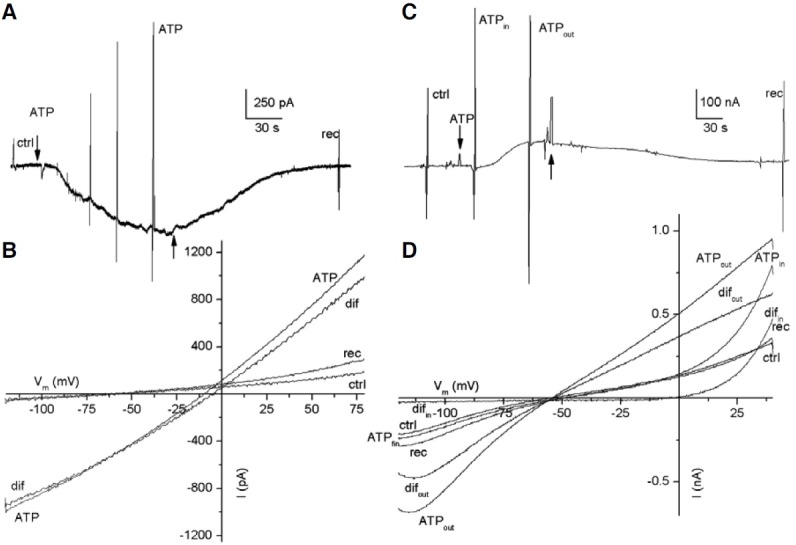
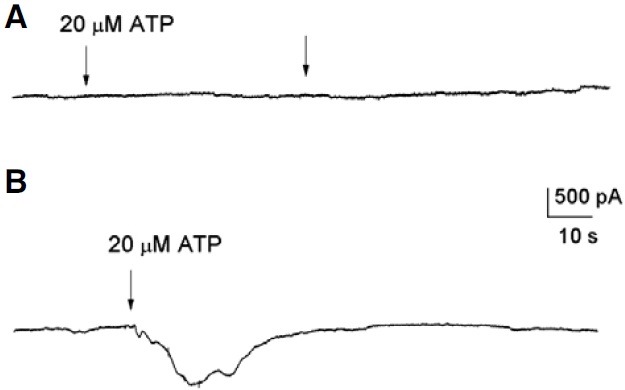
Figure 2A shows a sample recording of a biphasic inward current activated by ATP in a FC, in which the Fin- and Sin- currents reached 90 and 570 pA, respectively. This current returned to base line after ATP was removed from the bath. In this case, the I-V relationship showed an increase in the amplitude of both the inward and outward currents. For instance, the inward current increased from 50 to 974 pA at -120 mV, and the outward current from 189 to 1170 pA at 80 mV, in the presence of ATP (Fig. 2B). Also, ATP shifted the reversal potential of the current from -60 to -10 mV, toward the equilibrium potential of chloride (Fig. 2B, ctrl, ATP). Thus, the net ATP effect was obtained by subtracting the current generated at the peak of the Sin-current (the third response to a voltage ramp, Fig. 2A) from that of the control current. This current reversed at -4 mV (Fig. 2B, dif), very near to the chloride equilibrium potential (-10 mV), suggesting that ATP induced a chloride conductance in the FC. To determine if chloride is the ion that generated this current, the chloride of the pipette was replaced by gluconate. In this condition we did not observe currents generated by ATP (Fig. 3A). In this example, two applications of ATP did not produce any evident effect on the FC (n = 3). In a second series of experiments, we substituted gluconate for all the chloride in the bath but not in the recording electrode; under this condition an inward current was produced in response to ATP (Fig. 3B).
Fig. 3. Recordings obtained in chloride-free medium. Arrows indicate a 3-s application of ATP. (A) The patch pipette solution contained gluconate, and Ringer solution was used in the perfusion system. (B) The patch pipette solution contained chloride, and the perfusion system was gluconate-based Ringer solution.
Based on the earlier experiment, we chose to apply ATP in freshly dissociated nude oocytes (from two frogs) to investigate if the FCs show electrical responses similar to those of the oocytes. It was found that in oocytes, ATP induced Fin-currents of 85 ± 56 nA (n = 23), Sin-currents of 124 ± 57 nA (n = 7), and outward currents of 71 ± 38 nA (n = 9). Figure 2C shows a sample recording of an oocyte illustrating a Fin-current elicited by ATP followed by an outward current. Importantly, these outward currents were observed only in 40% of the oocytes tested. Analysis of the I-V relationships obtained from oocytes during the ATP responses showed that the Fin-currents increased sharply at values more positive than 0 mV (Fig. 2D). Consequently, the oocyte membrane increased its conductance compared to its corresponding control. Thus, the net ATP currents reversed near -4 mV (Fig. 2D, difin), suggesting a chloride conductance was activated during the Fin-currents.
Additionally, the whole current produced during the outward current activated by ATP in the oocyte was increased when evaluated using a voltage-ramp protocol (Fig. 2C, ATPout). For example, the inward response increased from 200 to 685 nA at -120 mV, and the outward current from 320 to 944 nA at 40 mV (Fig. 2D, ATPout). In this case, the net ATP current reversed near the equilibrium potential for potassium (-55 mV), suggesting the involvement of a K+ current (Fig. 2D, difout) during the outward current induced by ATP.
In several FCs, ATP induced oscillating inward currents (n = 6). Thus, the perforated patch-clamp technique was used to reduce the effect of intracellular metabolism (Marty and Neher, 1995). Unexpectedly, all the FCs tested with ATP using this configuration elicited oscillating inward currents. An example of this type of current is illustrated in Fig. 4A. In this recording, the current showed a maximal response of 92 pA. Interestingly, the second application of ATP on the same FC produced an oscillatory current that remained active even after the ATP was removed from the bath, but after a few seconds, the current did return to base line.
Fig. 4. Oscillating currents activated by ATP in FC. In (A), the FC was held at -60 mV; (B) illustrates the I-V relationship. In (A), the vertical lines show membrane currents activated by voltage ramps. In (A) and (C) the up arrows indicate the application of ATP. (B) ctrl, rec, are the control and recovery ramps; ATP represents the voltage ramp applied during the inward current; dif is the net effect induced by ATP. (C) Oscillating outward current generated by ATP in a FC held at 10 mV.
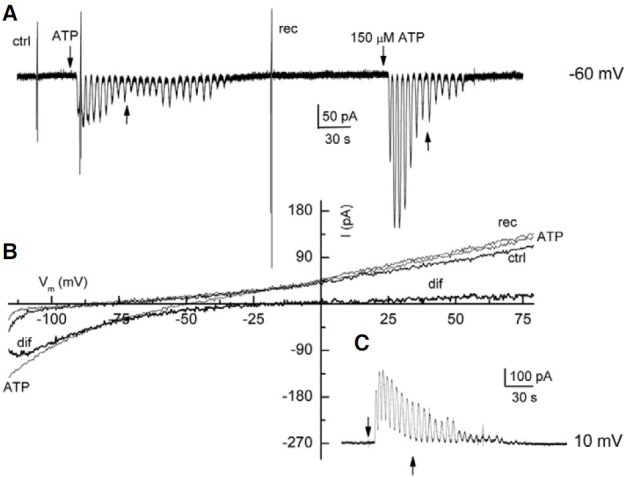
Experiments were designed to determine the ion responsible for the oscillatory current. Voltage-ramps were applied before, during, and after the oscillating inward current activated by ATP. Additionally, we changed the holding membrane potential of the FC from -60 to 10 mV. Therefore, if the current generated by ATP was carried by chloride, the current should eventually invert direction, i.e. it should become an outward current. A sample recording is shown in the Fig. 4. The analysis of the I-V relationship of this experiment revealed that ATP, at -120 mV, increased the amplitude of the inward current from 46 to 137 pA, whereas the outward current remained practically unaltered (Fig. 4B, ctrl, ATP). In addition, ATP shifted the reverse potential of the current from -75 to -48 mV, generating a net ATP-current that reversed at -12 mV, near the equilibrium potential for chloride (-10 mV) (Fig. 4B, dif). As expected, changing the holding potential of the cell caused ATP to elicit an outward current. Figure 4C shows a typical response of three experiments in which a FC held at 10 mV responded to ATP by generating an oscillating outward current. This result strongly suggested that the current was carried by chloride. It was particularly interesting that the membrane oscillated for 30-60 s after ATP was removed from the bath.
In another set of experiments, we investigated whether ATP modified the voltage-activated K+ currents in the FCs by applying ATP during voltage-pulse protocols known to activate these K+ currents. It was found that the outward K+ currents were blocked, whereas the inward currents were potentiated (Fig. 5A, ctrl, ATP). These responses partially recovered after the ATP was removed from the bath (Fig. 5A, rec). In this case, the current induced by ATP reached 323 pA and returned to base line after the ATP was removed from the bath (Fig. 5B). Further analysis of the I-V relationship revealed that during the ATP response, the outward K+-currents were blocked, and the inward currents were increased, both effects were observed from -120 to 80 mV (Fig. 5C, ctrl, ATP). Interestingly, the current did not reverse at any voltage tested. Thus, these experiments suggested that ATP (Fig. 5C, dif) blocked the outward K+-currents from 20 to 80 mV, and potentiated the inward currents from -120 to 80 mV.
Fig. 5. ATP-current analyzed using I-V relationships. (A) Currents activated by voltage-pulses in a FC. Ctrl, rec, are control and recovery currents, respectively; ATP indicates currents activated by voltage-pulses in the presence of ATP. In (B), the arrows indicate the application of 200 μM ATP; the vertical lines are voltage pulses applied before, during, and after the ATP response. (C) I-V relationship, dif refers to the net effect produced by ATP.
Angiotensin II responses
Ang II is an octapeptide that plays an important role in the oocyte’s maturation process, but little is known about the electrical signal that it induces in the FCs. Hence, we tested if Ang II would induce ionic currents in the cultured FC itself.
Application of 1 μM Ang II to 62 FC held at -60 mV yielded 10 smooth-inward currents of 157 ± 45 pA. Here we show sample recordings of inward currents elicited by Ang II in the FC. In Figs. 1E and 6A, the Ang II-induced an inward current that reached 80 pA and began to decay even before removal of Ang II. In general, Ang II generates smaller inward currents than ATP in the FCs.
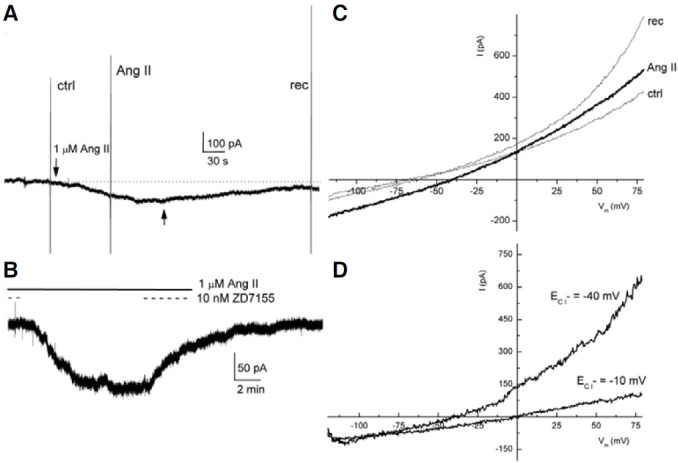
Fig. 6. Ang II responses produced in the FC, and I-V relationship. In (A), the vertical lines show membrane currents induced by voltageramp protocols; the arrows indicate the application of Ang II. In (B), the solid line indicates the continuous application of Ang II, and the dashed line shows the times of antagonist application. (C) and (D) are the I-V relationships. (C) ctrl, rec, are control and recovery ramps, respectively; Ang II is the voltageramp applied during the Ang II response; (D) ECl- = 0 mV is the net effect induced by Ang II in (A) ECl- = -40 mV is the net effect induced by Ang II when the chloride equilibrium potential was changed to -40 mV.
The typical Ang II responses elicited in follicles and ooctyes are oscillatory chloride inward currents, as illustrated in Fig. 1F (Matute et al., 1994). In this sample recording, Ang II induced a maximal current of 180 nA that remained active even when Ang II was removed from the bath. Thus, the inward currents elicited by Ang II in the FC contrasted with the oscillatory characteristics of the currents induced in the follicles and oocytes, although they both move chloride.
The effects produced by Ang II in the follicles and oocytes are mediated by activation of the AT1 receptor (Arellano et al., 1996; Matute et al., 1994). For this reason, we decided to test the specific AT1 receptor antagonist ZD7155 in the FC and also to change the equilibrium potential of chloride to -40 mV. In this experiment, Ang II was applied for 16 min in a FC (Fig. 6B, continuous line), which generated an inward current that reached 124 pA. When Ang II and ZD7155 were co-applied (Fig. 6B, dashed line), we did not find evidence of any induced ion current. Then, the antagonist was removed from the bath while Ang II remained, which elicited an inward current that was reversibly blocked by 10 nM ZD7155 (n = 3). A voltage-ramp protocol was introduced before, during, and after the Ang II response. It was found that Ang II increased both the inward and outward currents as compared to the I-V relationships obtained in the control solution (n = 3) (Fig. 6C, Ang II, ctrl). For instance, in the example shown, the inward current increased from 86 to 178 pA at -120 mV, and the outward current from 423 to 528 pA at 80 mV. It was also noticed that the voltagedependent currents were higher after the application of Ang II (Fig. 6C, ctrl, rec). Additionally, Ang II shifted the reversal potential of the current from -60 to -40 mV, towards the equilibrium potential of chloride (Fig. 6C, Ang II). Thus, the net Ang II effect (obtained by subtracting the current generated in the presence of Ang II from the control current), revealed a current that reversed near the equilibrium potential of chloride (-10 mV) (Fig. 6D, ECl = -10 mV). As mentioned before, the equilibrium potential of chloride was moved experimentally to -40 mV (see section corresponding to Electrophysiology). In consequence, the net Ang II-current reversed at the expected equilibrium potential of chloride (Fig. 6D, ECl = -40 mV). These experiments demonstrated that in the FC, Ang II generated a smooth chloride current by activating AT1 receptors.
Together, these results suggest that ATP and Ang II generate chloride currents in cultured FCs as they do separately in follicles.
DISCUSSION
It is well known that the ovary has a local renin-angiotensin system, in which Ang II plays a crucial role in the processes of ovulation and oocyte maturation (Yoshimura et al., 1992). Here, we show that Ang II generates ion currents in isolated FCs in culture. So far, there is only scattered information related to the electrical currents generated by Ang II in FCs separated from the oocyte, although it is well accepted that the entire follicle responds electrically to Ang II (Arellano et al., 1996). We do not present a basic mechanism by which the chloride current is activated by Ang II in FCs, but it might involve intracellular cascades similar to those in the whole follicles (Arellano et al., 1996), because the FC and oocyte membranes diffuse signals and second messengers, such as Ca2+ and IP3, through gap junctions (Miledi and Woodward, 1989; Sandberg et al., 1990). Thus, Ang II could potentially induce the production of IP3 in the FC, and consequently, the release of intracellular Ca2+ from intracellular stores to finally activate a calcium-dependent chloride current (Matute et al., 1994; Miledi, 1982; Sandberg et al., 1990).
Ang II responses were relatively rare in isolated follicular cells (∼16%). Thus, it may be that most Ang II responses found in follicles are mainly due to Ang II AT1 receptors located in the oocyte membrane (Arellano et al., 1996; Woodward and Miledi, 1991). In follicles, Ang II activates an oscillating inward current, mainly carried by chloride ions, by stimulating the inositol phosphate/ Ca2+-dependent chloride channel (Matute et al., 1994; Miledi et al., 1989). This response could be coupled to the FCoocyte complex through gap junctions, since the connexins involved permit the access of small molecules and Ca2+ that influence this type of response (Sandberg et al., 1990).
In cultured FCs, Ang II induced smooth chloride currents through activation of AT1 receptors; in contrast, in the follicle the currents activated by Ang II are oscillatory (Matute et al., 1994; Woodward and Miledi, 1991). Acting on the receptors of either the FCs or the oocyte, Ang II generates second messengers that can pass through through gap-junctions connecting the cells of the follicular complex (Miledi, 1982; Sandberg et al., 1990), thus influencing some of the oocyte processes, such as maturation.
ATP induced chloride currents in cultured FCs. Most of the electrophysiological studies performed in FCs have focused on KATP currents, which are blocked by sulphonylureas and pinacidil derivatives (Guillemare et al., 1995). The present study revealed that ATP currents (Fin and Sin) are more abundant (∼60%) in the cultured FCs than in defolliculated oocytes, since ATP responses were lost after defolliculation (Arellano et al., 1996). Thus, activation of ATP currents that originated in the FC membranes could induce the production of second messengers that travel through gap junctions between the FC and the oocyte membranes (Sandberg et al., 1990).
ATP responses obtained in FCs show characteristics similar to those observed in follicles (Arellano et al., 1996; Miledi et al., 1989), in which ATP induces a fast, inward chloride current (Fin-current). This process is followed by a slow, Ca2+-independent, inward chloride current (Sin-current) that depends on the FCs and their coupling to the oocyte membrane (Arellano et al., 1996; Miledi et al., 1989).
The results presented here strongly suggest that the ATP responses found in the follicles originate in the enveloping FC monolayer, as has been proposed for ATP-eliciting K+ currents in follicles (Arellano et al., 1996). It is important to emphasize that ATP blocked voltage-dependent K+ currents in the FC membranes. However, only five FCs showed outward K+ currents associated with a decrease in the membrane conductance (data not shown) (Sout, see Arellano et al., 1996). This could be explained by the variety of ATP receptor subtypes that can potentially be expressed in the FC membranes (Arellano et al., 1998). It was of particular interest to find that ATP induced mainly chloride currents in the FCs, whereas in the epithelial cells, ATP generated cationic currents, even when chloride ions were replaced by aspartate (Wu and Mori, 1999). Our observations suggest the existence of cell type-dependent roles for ATP. The balance of ions across the membrane is important throughout oogenesis, and hyperpolarization of the oocyte plasma membrane by activation of ATP receptors may contribute to restarting meiosis (Fujita et al., 2001; Wibrand et al., 1992). In addition, the activation of ATP receptors can depress the facilitating action of cAMP, a second messenger produced in the FCs by several stimuli (e.g. adenosine, FSH). Thus, the depression of the K+ currents by ATP in the FC could lead to maintaining the resting membrane potential, thus controlling hyperpolarization of the oocyte membrane. The main effect produced by ATP found in this study was the activation of a chloride current that depolarizes the FC membranes and can, therefore, play important roles in diverse physiological states of the oocyte.
In summary, this study shows that ATP and Ang II generate ion currents in FCs in culture. The results presented provide a better understanding of how these substances participate in the physiology of the FC, and potentially in the maturation of the oocyte.
Acknowledgments
We are grateful to Martín García-Servín and Efrén Ruíz-Alcíbar for taking care of the frogs, and to MSc. Leonor Casanova Rico and Bertha Esquivel Quiroz for their technical advice. We thank Dr. D.D. Pless for editing the manuscript. This work was supported with the Grants provided by the Dirección General de Asuntos del Personal Académico UNAM, IN 224604 to J.G.C.; Consejo Nacional de Ciencia y Tecnología (CONACYT), México G2575N to J.G.C. and R.M.; and 35033N to J.G.C. IN 202609 and IN 205308 to RM. M.M-H held stipends from CONACYT and CIAD AC.
References
- 1.Arellano R., Woodward R., Miledi R. Ion channels and membrane receptors in follicle-enclosed Xenopus oocytes. In Ion Channels, T. Narahashi, ed. Plenum Press; New York: (1996). pp. 203–259. [DOI] [PubMed] [Google Scholar]
- 2.Arellano R.O., Garay E., Miledi R. Cl- currents activated via purinergic receptors in Xenopus follicles. Am. J. Physiol. (1998);274:C333–340. doi: 10.1152/ajpcell.1998.274.2.C333. [DOI] [PubMed] [Google Scholar]
- 3.Fujita R., Kimura S., Kwasaki S., Takashima K., Matsumoto M., Hirano H., Sasaki K. ATP suppresses the K+ current responses to FSH and adenosin in the follicular cells of Xenopus oocyte. Jpn. J. Physiol. (2001);51:491–500. doi: 10.2170/jjphysiol.51.491. [DOI] [PubMed] [Google Scholar]
- 4.Guillemare E., Lazdunski M., Honoré E. Glibenclamide opens ATP-sensitive potassium channels in Xenopus oocyte follicular cells during metabolic stress. Mol. Pharmacol. (1995);47:588–594. [PubMed] [Google Scholar]
- 5.Hamill O.P., Marty A., Neher E., Sakmann B., Sigworth F.J. Improved patch clamp techniques for high resolution current recording from cells and cells free membrane patches. Pflug. Arch. (1981);391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 6.Honoré E., Lazdunski M. Single-channel properties and regulation of pinacidil/glibenclamide-sensitive K channels in follicular cells from Xenopus oocyte. Pflug. Arch. (1993);424:113–121. doi: 10.1007/BF00374601. [DOI] [PubMed] [Google Scholar]
- 7.King B.F., Pintor J., Wang S., Ziganshin A.U., Ziganshina L.E., Burnstock G. A novel P1 purinoreceptor activates an outward K+-current in follicular oocytes of Xenopus laevis. J. Pharmacol. Exp. Ther. (1996);276:93–100. [PubMed] [Google Scholar]
- 8.Marty A., Neher E. Tight-seal whole-cell recording. In Single-Channel Recording, E. Neher and B. Sakmann, eds. Plenum Press; New York: (1995). p. 45. [Google Scholar]
- 9.Matute C., Pulakat L., Río C., Valcárcel C., Miledi R. Properties of angiotensin II receptors in glial cells from the adult corpus callosum. Proc. Natl. Acad. Sci. USA. (1994);91:3774–3778. doi: 10.1073/pnas.91.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc. R. Soc. Lond. B Biol. Sci. (1982);215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 11.Miledi R., Woodward. R.M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J. Physiol. (1989);416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miledi R., Parker I., Sumikawa K. Transplanting receptors from brains into oocytes, In: Fidia Research Foundation Neuroscience Award Lectures. Raves Press; New York: (1989). pp. 57–90. [Google Scholar]
- 13.Neher E., Sakmann B. Single-channel recording. Plenum Press; New York: (1995). [Google Scholar]
- 14.Oldham A.A., Allott C.F., Major J.S., Pearce R.J., Roberts D.A., Russell S.T. Zeneca ZD 7155. A novel, potent and orally-effective angiotensin II receptor antagonist. Br. J. Pharmacol. (1993);109(S):136P. [Google Scholar]
- 15.Sandberg K., Bor M., Ji H., Markwick A., Millan M.A., Catt K.J. Angiotensin II-induced calcium mobilization in oocytes by signal transfer through gap junctions. Science. (1990);249:298–301. doi: 10.1126/science.2374929. [DOI] [PubMed] [Google Scholar]
- 16.Wibrand F., Honoré E., Lazdunski M. Opening of glibenclamide-sensitive K+ channels in follicular cells promotes Xenopus oocyte maturation. Proc. Natl. Acad. Sci. USA. (1992);89:5133–5137. doi: 10.1073/pnas.89.11.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodward R.M., Miledi R. Angiotensin II receptors in Xenopus oocytes. Proc. R. Soc. Lond. B. (1991);244:11–19. doi: 10.1098/rspb.1991.0044. [DOI] [PubMed] [Google Scholar]
- 18.Wu D., Mori N. Extracellular ATP-induced inward current in isolated epithelial cells of the endolymphatic sac. Biochim. Biophys. Acta. (1999);1419:33–42. doi: 10.1016/s0005-2736(99)00053-x. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura Y., Karube M., Koyama N., Shiokawa S., Nanno T., Nakamura Y. Angiotensin directly induces follicle rupture and oocyte maturation in the rabbit. FEBS Lett. (1992);307:305–308. doi: 10.1016/0014-5793(92)80701-h. [DOI] [PubMed] [Google Scholar]


