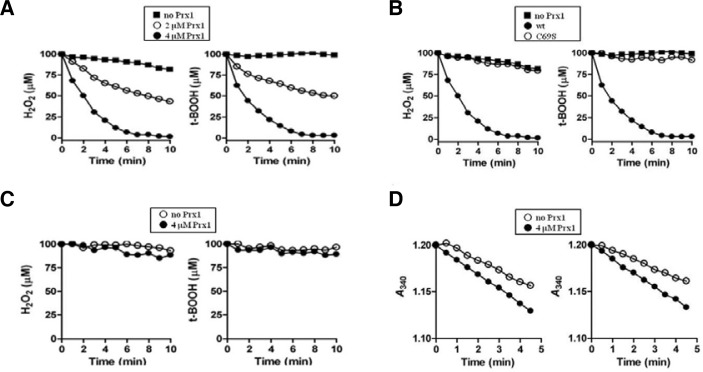
Fig. 2.
Peroxidase activity of Prx1. Mean values of triplicate experiments are shown. (A) DTT-dependent removal of H2O2 (left panel) and t-BOOH (right panel) by Prx1. Peroxidase reaction was carried out at room temperature in 200 μl of reaction mixture containing 50 mM HEPES (pH 7.0), 5 mM DTT, 100 μM H2O2 or 100 μM t-BOOH, and in the absence (closed square) or presence of Prx1 (closed circle, 2 μM; closed triangle, 4 μM). At the indicated times, the remaining peroxides were measured using FOX1 reagent as described in Materials and methods. (B) Peroxidase reaction was carried as in (A) with 100 μM H2O2 (left panel) or 100 μM t-BOOH (right panel) in the absence (closed square) or presence of 4 μM Prx1 (wild type, closed circle; C69S, closed triangle). (C) Peroxidase reaction was performed using 4 μM Prx1 as in (A), except the presence of GSH instead of DTT as a reducing equivalent: closed square, without Prx1; closed triangle, with Prx1. (D) Peroxidase activity linked to NADPH oxidation with S. cerevisiae Trx system was monitored by decreases of A340 in a 200-μl reaction mixture containing 50 mM HEPES (pH 7.0), 0.2 mM NADPH, 2 μM ScTrr1, 5 μM ScTrx1, 4 μM Prx1, and either 0.1 mM H2O2 (left panel) or 0.1 mM t-BOOH (right panel): closed square, without Prx1; closed triangle, with Prx1.