Abstract
Osteocytes that have a dendritic appearance are widely believed to form a complex cellular network system and play crucial roles in mechanotransduction as a principal bone mechanosensor, which is the basis of their neuronal-like biology, as previously reported. Neuropeptide Y (NPY) and reelin mRNA, which are brain-specific neurogenic markers, have been identified in osteocytes. However, changes in the production of NPY and reelin in response to specific biochemical stimulation are unknown. In this study, we investigated the in vitro effect of corticosterone, one of the endogenous glucocorticoids, on the expression of NPY and reelin in the MLO-Y4 osteocyte cell line. Cells were treated with corticosterone at different concentrations (10−9 M−10−5 M) for 1, 3, 6, 12 and 24 h. As revealed, corticosterone reduced the MLO-Y4 cell viability and proliferation in a dose- and time-dependent manner based on an MTT assay and a Vi-CELL analyzer. The cells were then incubated with corticosterone (10−6 μM), and the NPY and reelin expression levels were detected at 1, 3, 6, 12 and 24 h using real-time PCR and Western blot analysis. These results demonstrated that at the gene and the protein levels, corticosterone significantly upregulated the NPY and reelin expression in a time-dependent manner. The application of a glucocorticoid receptor antagonist, RU486, reversed the reduced cell viability and the increased expression of NPY and reelin that were caused by corticosterone. To the best of our knowledge, this is the first report to verify that corticosterone regulates the NPY and reelin expression in osteocytes.
Keywords: corticosterone, NPY, osteocytes, reelin
INTRODUCTION
The popular theory concerning osteocytes, which are the most abundant cells in an adult skeleton (Lanyon, 1993), is that osteocytes function as mechanosensors directing bone remodeling and regulators modifying mineralization and mineral metabolism (Bonewald, 2006). Moreover, the dendritic morphology and mechanotransduction of osteocytes and the amazing osteocytic network (Bonewald and Johnson, 2008; Papachroni et al., 2009; Rho et al., 2004) also define their neuronal-like biology (Marotti, 2000). Evidence of the expression of neurogenic markers and several neurotransmitter-related receptors (Paic et al., 2009; Westbroek et al., 2001) suggested that osteocytes serve as a mechanosensor or a neuronal-like regulator of bone mass. It provides a fresh perspective for investigating the mechanisms through which osteocytes recognize various stimuli and coordinate the activities of osteoblasts and osteoclasts.
Recently, two brain neurogenic markers, namely neuropeptide Y (NPY) and reelin, have been identified as having higher mRNA levels in osteocytes than osteoblasts (Paic et al., 2009). NPY, a 36-amino acid peptide neurotransmitter found in the brain and the autonomic nervous system, acts as a traditional neuronal regulator of energy homeostasis (Chronwall et al., 1985). NPY is a potential modulator of bone remodeling. It exerts hypothalamic actions on the bone and adipose tissues and locally affects osteoblasts and adipocytes (Karsenty, 2000; Zengin et al., 2010). Recent studies have demonstrated NPY expression in osteocytes and an inhibitory effect of NPY on the osteoblast activity (Igwe et al., 2009; Paic et al., 2009). These results indicate that osteocytic NPY could be a potential mediator for the functions of osteocytes and osteoblasts. Due to the load-responsive nature of local NPY expression (Igwe et al., 2009), exploring the expression dynamics of osteocytes in response to bone catabolic or anabolic stimuli is therefore necessary.
Reelin, a large secreted extracellular matrix (ECM) glycoprotein (D’Arcangelo et al., 1995), plays a pivotal role in brain development (Dulabon et al., 2000) and the adult brain (Niu et al., 2008). Peripheral reelin mRNA is found in various tissues and cells including the adult mammalian blood, liver, eyes, odontoblasts, osteoblasts, and osteocytes (Kobold et al., 2002; Maurin et al., 2004; Pulido et al., 2007; Smalheiser et al., 2000). The physiological significance of reelin in bone cells is unknown, but earlier studies have suggested a site-specific expression in limb and skull bones/cells and a possible link between reelin and abnormal bone remodeling in the otic capsule (Rawlinson et al., 2009; Schrauwen et al., 2009). Notably, in osteoblast-like cells, the expression of reelin can be dramatically downregulated by certain biological materials and BMP-2 (van der Zande et al., 2010). This research article will provide more evidence for the gene and protein expression of reelin in osteocytes.
In both nervous and non-nervous tissues and cells, NPY and reelin are sensitive to dexamethasone and corticosterone exposure, which can induce bone loss systemically and/or locally (Akabayashi et al., 1994; Lussier et al., 2011; Weinstein et al., 1998). An exogenous glucocorticoid (GC), dexamethasone, has been widely used for the treatment of inflammatory and autoimmune diseases (O’Brien et al., 2004). Corticosterone in rodents and cortisol in humans, which are endogenous GCs, are produced in response to the acute and chronic stresses (Djordjevi et al., 2003). Compared with dexamethasone, the effects of endogenous corticosterone/cortisol on bone cells are closer to their activities in vivo. In this study, we have explored the effects of corticosterone (CORT) on the expression of NPY and reelin in osteocytes by using the MLO-Y4 cell line as an example.
MATERIALS AND METHODS
Chemicals
Corticosterone (Sigma, USA) was dissolved in fetal bovine serum (Invitrogen, USA) to a concentration of 1 mM and stored at −20°C. The diluted samples were freshly made at a concentration range of 10−9−10−5 M. The glucocorticoid receptor (GR) antagonist RU486 (Sigma, USA) was dissolved in ethanol to a concentration of 10 mM, and a working solution was freshly prepared at a concentration of 10 μM.
Cell culture
The MLO-Y4 cells were generously given by Prof. Lynda F. Bonewald from the Department of Oral Biology, University of Missouri in Kansas City, USA. The cells were cultured in collagen-coated (rat tail collagen type I; 0.15 mg/ml in 0.02 N Acetic acid) flasks in an α-modified essential medium (α-MEM; Gibco, USA) supplemented with 5% fetal bovine serum and 5% calf serum (HyClone, USA) at 37°C in a humi-dified atmosphere with 5% CO2, as previously described (Chen et al., 2010). The medium was replaced every two days.
Immunofluorescence
For immunostaining of the osteocytes in vitro, the cells were grown on glass cover slips, washed with phosphate-buffered saline (PBS; pH 7.4), fixed for 20 min in 4% paraformaldehyde, and washed and blocked with a 5% goat serum at 37°C for 20 min. The cells were subsequently stained with a mouse anti-reelin (1:150; Chemicon; MAB5364) or a rabbit anti-NPY (1:150; Santa Cruz; sc-28943) antibody, which was followed by a DyLight™ 594-conjugated anti-mouse or a DyLight™ 488-conjugated anti-rabbit IgG (1:100; Zhongshan Golden Bridge; ZF-0413/ZF-0411) for immunofluorescence detection. Additionally, the cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Photographs were taken using a fluorescence microscope (Olympus, Japan).
Cell viability assay
MLO-Y4 cells were seeded in 96-well plates, and their cell growth was arrested by a 12-h incubation in serum-free medium. The cells were then treated with CORT at the indicated concentrations (0, 10−9 M, 10−8 M, 10−7 M, 10−6 M and 10−5 M) in α-MEM supplemented with 0.5% BSA for 0, 1, 3, 6, 12 and 24 h. To detect the effects of glucocorticoid receptors, RU486 was added 2 h prior to the addition of 1 μM CORT. The MTT assay data were measured by an absorbance at 490 nm using a microplate reader.
The MLO-Y4 cells were plated to a density of 3 × 105 cells/well in 6-well plates for confluency. The cells were then treated with CORT and/or RU486 as described above. Subsequently, they were harvested using 0.5% trypsin and three PBS washes. The cells were suspended in 1 ml of fresh culture medium and transferred to a Vi-CELL™ Cell Viability Analyzer (Beckman, USA), which was utilized to determine the number of cells that which have absorbed the trypan blue dye and which have not. The Vi-CELL will analyze up to 100 images for a determination of cellular viability. Each experiment was repeated at least three times.
Real-time PCR
The arrested cells were treated with 1 μM of CORT alone for 0, 1, 3, 6, 12 and 24 h in α-MEM supplemented with 0.5% BSA. In the experiments in which RU486 was used, the cells were treated with 0.1% ethanol alone (the vehicle), 10 μM RU486 alone (the RU486 group), or 10 μM RU486 followed by 1 μM CORT (the RU+ CS groups) for 1, 3, 6, 12 and 24 h. Total RNA (1 μg) was isolated from the cells using TRIzol Reagent (Invitrogen, USA) and reverse transcribed into cDNA using a PrimeScript® RT Reagent Kit (DRR037A; Takara, Japan) according to the manufacturers’ instructions. The cDNA synthesis was performed for 15 min at 37°C and 5 s at 85°C. The PCR amplification was performed using SYBR® Premix RX Taq™ II (DRR081A; Takara, Japan) at an initial melting temperature of 95°C for 30 s, which was followed by 40 cycles of 95°C for 5 s, 60°C for 31 s, and 72°C for 31s, and then a dissociation step was performed in the ABI PRISM® 7300 Fast Real-Time PCR System. The primer sets (mouse) used are as follows: reelin, forward 5′-ACC TGA CGC CCA CTG AGA ACT-3′; and reverse, 5′-CGG GTA AGC ACT GAG GGA CTA A-3′; NPY, forward 5′-GTA ACA AGC GAA TGG GGC TGT-3′; and reverse 5′-GTA GTG TCG CAG AGC GGA GTA GT-3′; DMP1, forward 5′-AGA GGG TAG AGG AAT CGC-3′; and reverse 5′-TGA CTT TCT TCT GAT GAC TCA CT-3′; GAPDH, forward 5′-GAC ATC AAG AAG GTG GTG AAGC-3′; and reverse 5′-GAA GGT GGA AGA GTG GGA GTT-3′. The fold change was calculated as follows: 2−ΔΔCt, in which, ΔΔCt = ΔCttreatment − ΔCtcontrol, ΔCt = Cttargetgene − CtGAPDH.
Western blot analysis
Following any treatments, the MLO-Y4 cells were washed three times with ice-cold PBS, lysed with a lysis buffer [50 mM Tris-HCl, 1 mM EDTA, 150 mM NaCl, 0.5% Triton X-100, 0.5% Nonidet P-40, 0.5% Na deoxycholate, and 0.1% SDS supplemented with 0.5% (v/v) leupeptin, 0.1% (v/v) aprotinin and 0.5% (v/v) 100 μM phenylmethylsulfonyl fluoride], and then centrifuged at 14,000 rpm at 4°C for 15 min at 4°C. The protein content was quantified using a BCA Protein Assay Kit (Pierce, USA). Equal amounts of protein were separated using 6%, 10% and 12% SDS-polyacrylamide gel electrophoresis for reelin, β-actin and NPY, respectively. The proteins were electrotransferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% non-fat dry milk in a TBS buffer containing 0.1% Tween-20 (TBST) for 1 h at room temperature. The membranes were subsequently incubated overnight with an anti-reelin monoclonal antibody G10 (1:200), an anti-NPY polyclonal antibody (1:200), or an anti-β-actin antibody (1:500) diluted in TBST at 4°C. After removing the unbound primary antibodies by performing three 10-minute washes with TBST, the membranes were incubated with horse-radish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (1:8000) diluted in TBST for 1 h at 37°C and were washed with three 10-minute washes with TBST. Chemiluminescence (CL) detection was performed to detect the antibody-associated protein bands using X-ray photographic films. A densitometric analysis of the immunoreactivity of the proteins was conducted using a Bio-Rad Image Analysis System.
Statistics
The results were expressed as the means ± standard deviation. The differences between the groups were tested by a one-way ANOVA using SPSS software. A value of p < 0.05 was considered statistically significant.
RESULTS
Expression of reelin and NPY in MLO-Y4 cells
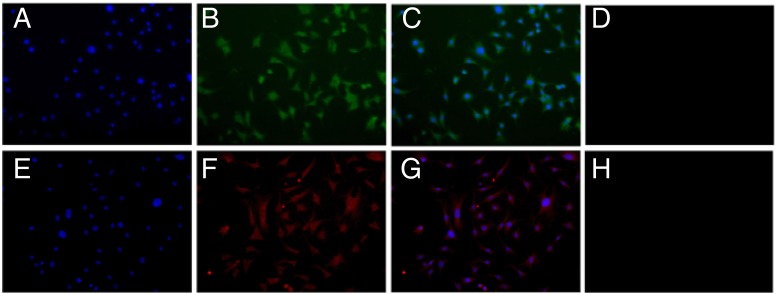
NPY immunoreactivity, which was detected with a rabbit polyclonal anti-NPY antibody by immunofluorescence (IF), was present in the MLO-Y4 cells with moderate staining in the cell bodies and reduced staining in the cell dendrites (Figs. 1A–1D). The reelin identification using IF also showed a low to medium staining in the MLO-Y4 cell bodies and a weaken staining in some of cell dendrites (Figs. 1E–1H).
Fig. 1.
Analysis of NPY (A-D) and reelin (E-H) expression in the MLO-Y4 cells by immunofluorescence. (A, E): Nuclear staining of the MLO-Y4 cells using DAPI (200×). (B, F): Immunofluorescence labelling of NPY (B) and reelin (F) performed with anti-NPY antibody and anti-reelin antibody respectively in MLO-Y4 cells (200×). (C, G): Nuclear staining mergered with the NPY (C) or reelin (G) immunostaining (200×). (D, H): Control staining of the MLO-Y4 cells without the primary antibody (200×).
Reduction of MLO-Y4 cell viability by CORT
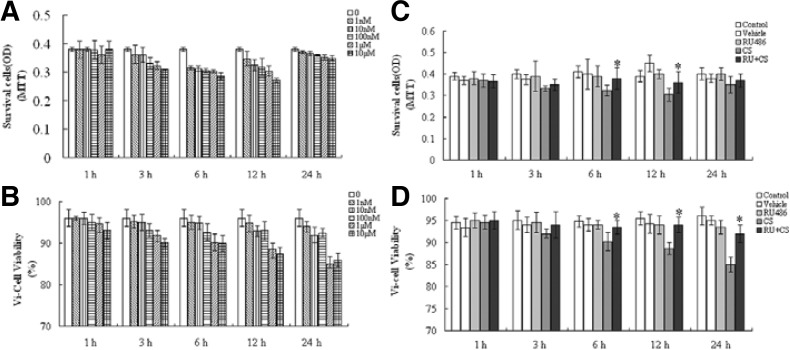
The MLO-Y4 cells were treated with various CORT concentrations (10−9−10−5 M) for 0, 1, 3, 6, 12 and 24 h after growth arrest using a serum-free medium, and then the cell viability was determined using an MTT assay and a Vi-Cell automated analyzer. The CORT exposure reduced the expected number and propotion of viable cells in a time- and dose-dependent manner compared with the control samples (Figs. 2A and 2B). This inhibitory effect was more obvious after the CORT applications of 10−6 M and 10−5 M for 3, 6 and 12 h. There was a rebound in the OD values at 24 h of CORT treatment in the MTT assay (Fig. 2A), which most likely suggests the recovery of the metabolic activity of the cells. To investigate whether the GR was involved in these inhibitory effects, RU 486 was added 2 h prior to the addition of 1 μM CORT. RU486 reversed the reduced viability that was caused by CORT (p < 0.05, Figs. 2C and 2D). Ethanol (0.1%) or RU486 alone did not affect the viability of the MLO-Y4 cells.
Fig. 2.
A reduction in MLO-Y4 cell viability by corticosterone. (A, B) The number and proportion of viable cells were detected using an MTT assay and a Vi-Cell™ cell viability analyzer, respectively. (C, D) Pretreatment with RU486 reversed the inhibitory effectts by CORT (1 μM) on the cell viability. All data shown are the means ± SD from triplicate tests (*p < 0.05, RU486 plus CORT vs. CORT-treated groups). CS = corticosterone, RU + CS = RU486 plus CORT.
CORT upregulated the NPY and reelin mRNA expression through GR
Following the CORT/RU486 treatment of the MLO-Y4 cells, the gene expression of dentin matrix acidic phosphoprotein 1 (DMP1) was detected using real-time PCR. These results demonstrated that the DMP1 expression was significantly increased in a time-dependent manner, especially at 3 and 6 h following the CORT treatment compared to the control (greater than 5-fold; p < 0.05; Fig. 3A). This increased effect, however, was completely inhibited by the RU486 pretreatment (p < 0.05, Fig. 3A). The positive effect of CORT on the NPY mRNA expression was noted after 1 h, which peaked at 6 h and then gradually decreased, but it was still superior to the control level (p < 0.05, Fig. 3B). The addition of RU486 decreased the increased expression of NPY that was caused by the CORT treatment (Fig. 3B). The reelin expression was induced at 1 h, returned to the basal level at 3 h and then was re-induced 6 h later (p < 0.05; Fig. 3C). The upregulation of reelin was completely inhibited by the pretreatment with RU486 (p < 0.05; Fig. 3C). These results suggested that CORT promoted the NPY and reelin mRNA expression in a GR-dependent way.
Fig. 3.
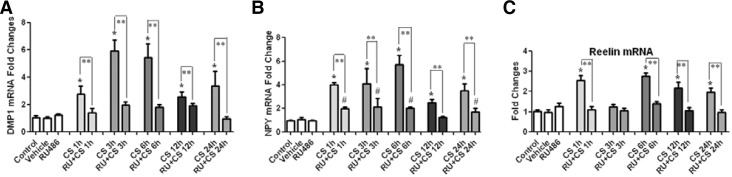
CORT-induced gene expression in the MLO-Y4 cells. (A) The expression of DMP1 was significantly increased in a time-dependent manner, which was abrogated by RU486. (B) CORT upregulated the expression of NPY mRNA, which was partially inhibited by pretreatment with RU486. (C) The reelin gene expression was induced by the application of CORT and reversed by the RU486 pretreatment. The results of the real-time PCR analysis with GAPDH as an endogenous control are shown as the means ± SD (n = 3; *p < 0.05 vs. the control; #p < 0.05 vs. the vehicle or RU486-treated group; **p < 0.05, RU486 plus CORT vs. the corresponding CORT-treated groups). CS = corticosterone, RU + CS = RU486 plus CORT.
CORT increased NPY and reelin protein expression by GR
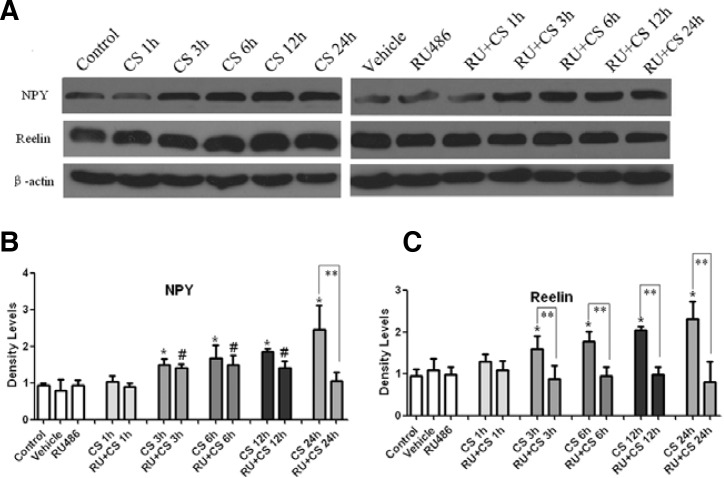
Western blot analysis was performed to determine protein expression of NPY and reelin in the MLO-Y4 cells following the treatments (Fig. 4A). A quantitative analysis of the protein-positive bands indicated that significantly increased levels of NPY were present with 3, 6, 12 and 24 h of CORT treatment (approximately 1.5-, 1.7-, 1.9- and 2.5-fold, respectively; p < 0.05, Fig. 4B). The increased NPY expression at 3, 6 and 12 h was weakened by the pretreatment with RU486 but remained superior to the vehicle or RU486-treated group (p < 0.05; Fig. 4B). The application of RU486 abrogated the CORT-induced NPY expression at 24 h (p < 0.05; Fig. 4B) .
Fig. 4.
CORT induced NPY and reelin protein production in the MLO-Y4 cells. The band intensities (A) were quantified with densitometry (B, C), which showed the expression of both proteins remained at the basal level at 1 h, but significantly increased from 3 to 24 h. RU486 partially reversed the CORT-induced NPY expression and abrogated the increased reelin expression. The results shown are the means ± SD (n = 3; *p < 0.05 vs. the control; #p < 0.05 vs. the vehicle or RU486-treated group; **p < 0.05, RU486 plus CORT vs. the corresponding CORT-treated groups). CS = corticosterone, RU + CS = RU486 plus CORT.
For reelin, only a single band was detected at ∼388 kDa in the MLO-Y4 cells (Fig. 4A), which was significantly enhanced after 3 h of CORT treatment in a time-dependent manner compared with the control (p < 0.05; Fig. 4C). RU486, however, abrogated these positive effects of CORT on the reelin expression. It implied that CORT upregulated the protein expression of reelin in a GR-dependent manner.
DISCUSSION
Osteocytes constitute more than 90–95% of the cells in an adult skeleton (Lanyon, 1993). Previous studies, which reported on the physical morphology and biological functions (Bonewald and Johnson, 2008, Papachroni et al., 2009), have implied the presence of an osteocytic neuronal-like biology. More interestingly, the expression of several neurogenic markers has been found in osteocytes. NPY and reelin, two neural tissue-related genes, have been shown to have high mRNA levels in osteocytes (Paic et al., 2009). In the present paper, we provide the first evidence that the gene and protein expression of reelin and NPY can be regulated by corticosterone, a catabolic factor for bone, in the MLO-Y4 cells.
As shown in Fig. 1, the expression of NPY was predominantly present in the cytoplasm surrounding the nuclei of the MLO-Y4 cells. The faint green fluorescence was shown in some of cell processes connected to the adjacent cells (Figs. 1B and 1C). NPY was also secreted into the culture medium by osteocytes, which was confirmed by an ELISA kit (unpublished data). Given its inhibitory effect on the osteoblast activity in vitro (Igwe et al., 2009), NPY may be a non-negligible biological factor between osteocytes and osteoblasts in vivo. The reelin immunoreactivity was detected with a low to medium staining in the MLO-Y4 cell bodies (Figs. 1E–1H). A faint reelin immunostaining was also detected in the typical dendrites of the MLO-Y4 cells (Figs. 1F and 1G). It has been well documented that reelin stimulates dendrite outgrowth and regulates the migration of neuroblasts in the nerve system (Niu et al., 2008). However, it remains unknown whether reelin contributes to the dendritic morphology of osteocytes.
CORT is a stress hormone and can mediate NPY and reelin production in the nervous system both in vivo and in vitro (Gross et al., 2010; Husum and Mathé, 2002). In the present study, the effectiveness of the CORT treatment in MLO-Y4 cells could be verified by the expression of DMP1, an ECM protein produced by osteocytes. Consistent with previous reports that dexamethasone can increase the mRNA level of DMP1 (Mikami et al., 2008), this study also demonstrated that the DMP1 gene expression could be significantly enhanced by corticosterone in osteocytes through GR.
Through an intervention, we have shown that the mRNA and protein levels of NPY were upregulated by CORT in osteocytes (Figs. 3 and 4), which corresponds with previous reports (McKibbin et al., 1992; Shimizu et al., 2008). A positive correlation between NPY and CORT was also found in certain feeding paradigms (Wang et al., 1998). In the present study, the addition of RU486 partially reversed the upregulation of NPY that was caused by CORT. This indicated that the GR mediated the CORT-induced NPY expression. GR is a widely expressed member of the nuclear receptor superfamily, which is fundamentally required for CORT-mediated actions. Corticosteroids modulate gene transcription via either the binding of GR homodimers to GC-responsive elements (GREs) present in the promoters of hormone-responsive genes or the protein-protein interactions of GR monomers with transcription factors such as NF-κB, AP-1, IRF-3, and STAT5 (Reichardt and Schütz, 1998). There are several transcription factor-binding sites in the mouse NPY gene promoter, including AP-1, AP-2 and Sp1, which can interact with GR to regulate gene transcription (Titolo et al., 2008). Although it has been demonstrated that glucocorticoid-responsive elements (GREs) are present in the far upstream region of the rat NPY gene (Misaki et al., 1992), there have been no reports about GREs in the mouse NPY gene. Therefore, we speculate that the binding of transcription factors to the NPY promoter is involved in the positive effects of CORT on the NPY gene expression.
CORT administration increased the reelin mRNA and protein levels in the MLO-Y4 cells (p < 0.05; Figs. 3 and 4), which is partially consistent with previous reports (Gross et al., 2010; Kobold et al., 2002). A negative regulation of CORT on reelin has also been noticed in murine hippocampi (Lussier et al., 2009; 2011). The application of RU486 abrogated the CORT-increased reelin expression, suggesting the presence of GR signaling cascades. The mouse reelin promoter has been shown to contain potential binding sites for Sp1 and AP-2 (Royaux et al., 1997). Whether the reelin promoter contains GREs is presently unknown. Therefore, the exact mechanism leading to the corticosterone-mediated induction of the NPY and reelin genes remains undefined.
It is interesting that CORT increased the NPY and reelin gene expression (Figs. 3B and 3C) while the cell viability was reduced in the present study (Fig. 2). Based upon the mechanisms of GR actions, which have been documented previously (Necela and Cidlowski, 2004), CORT appears to decrease the MLO-Y4 cell viability through a complex process of signaling cascades that began with GR-mediated changes in gene expression through transactivation and transrepression. Thus, the question arises of whether there is any possible casual relationship between the cell viability and NPY/reelin gene expression. In addition, CORT most likely repressed transcription factors and cytokines that were required for the survival of MLO-Y4 cells via the repression of NF-κB and AP-1 signaling and the regulation of antiapoptotic and proapoptotic Bcl-2 members (Necela and Cidlowski, 2004). Therefore, the underlying mechanism of the CORT-reduced cell viability is an interesting topic.
The role of NPY as one of major regulators of bone homeostasis is mainly manifested through a central control via the hypothalamic Y2 receptor and a peripheral pathway via the local Y1 receptor (Allison et al., 2006; Teixeira et al., 2009). Combined with the results of this experiment, it is plausible that an increase in the serum corticosterone concentration induces the NPY expression systemically and locally in vivo. Therefore, the local NPY pathway is expected to provide potential therapeutic targets for the treatment of glucocorticoid-induced osteoporosis. Little is known about the effects of reelin; therefore, in vitro odontoblast studies in which a colocalization of the nerve fibers with reelin was observed can be instructive (Maurin et al., 2004). The nerve fibers enter the bone with blood vessels and then branch to form rings around the osteoblasts and osteocytes (Sherman, 1963). Direct neurite-osteoblastic and neurite-osteoclastic cell communications have been identified by in vitro co-culture systems (Obata et al., 2007; Suga et al., 2010). Being approximately 10 times and 100 times greater than osteoblasts and osteoclasts respectively, osteocytes are more likely to contact or communicate with nerve terminals, and reelin serves as one possible mediator.
Taken together, the expression of NPY and reelin appear to be a common feature between the nervous system and bones. Increased serum corticosterone in mice or cortisol in humans can alter the expression of NPY and reelin in the nervous system (Akabayashi et al., 1994; Gross et al., 2010; Husum and Mathé, 2002; Lussier et al., 2011) and possibly in bone. Although the exact role of reelin still remains unclear, the significance of NPY in bone remodeling has been recognized. The above findings may not only contribute to the development of potential therapies for bone diseases, but also may enhance the understanding of osteocytic neuronal-like biology and the connection between the nervous and skeletal systems.
Acknowledgments
We thank Prof. L.F. Bonewald from the Department of Oral Biology, University of Missouri in Kansas City, Missouri, USA, for the gift of the MLO-Y4 cells. This work was supported by the National Natural Science Foundation of China (No. 30500568 and No. 81170941), the Program for New Century Excellent Talents at the University (NCET-08-0375), and the Science & Technology Support Project, Science and Technology Department of the Sichuan Province (No. 2009FZ0062 and No. 2011SZ0157).
REFERENCES
- Akabayashi A., Watanabe Y., Wahlestedt C., McEwen B.S., Paez X., Leibowitz S.F. Hypothalamic neuropeptide Y, its gene expression and receptor activity: relation to circulating corticosterone in adrenalectomized rats. Brain Res. 1994;665:201–212. doi: 10.1016/0006-8993(94)91339-0. [DOI] [PubMed] [Google Scholar]
- Allison S.J., Baldock P., Sainsbury A., Enriquez R., Lee N.J., Lin E.J.D., Klugman M., During M., Eisman J.A., Li M. Conditional deletion of hypothalamic Y2 receptors reverts gonadectomy-induced bone loss in adult mice. J. Biol. Chem. 2006;281:23436–23444. doi: 10.1074/jbc.M604839200. [DOI] [PubMed] [Google Scholar]
- Bonewald L.F. Mechanosensation and transduction in osteocytes. Bonekey Osteovision. 2006;3:7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald L.F., Johnson M.L. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Ma Y., Ye H., He Y., Li X., Li J., Zhu Z., Wang H. ERK1/2 is involved in cyclic compressive force-induced IL-6 secretion in MLO-Y4 cells. Biochem. Biophys. Res. Commun. 2010;401:339–343. doi: 10.1016/j.bbrc.2010.09.044. [DOI] [PubMed] [Google Scholar]
- Chronwall B.M., DiMaggio D.A., Massari V.J., Pickel V.M., Ruggiero D.A., O’Donohue T.L. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G., Miao G.G., Chen S.C., Scares H.D., Morgan J.I., Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Djordjevi J., Cviji G., Davidovi V. Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiol. Res. 2003;52:67–72. [PubMed] [Google Scholar]
- Dulabon L., Olson E.C., Taglienti M.G., Eisenhuth S., McGrath B., Walsh C.A., Kreidberg J.A., Anton E.S. Reelin binds alpha 3 beta 1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- Gross C.M., Flubacher A., Tinnes S., Heyer A., Scheller M., Herpfer I., Berger M., Frotscher M., Lieb K., Haas C.A. Early life stress stimulates hippocampal reelin gene expression in a sex-specific manner: Evidence for corticosterone-mediated action. Hippocampus. 2010;22:409–420. doi: 10.1002/hipo.20907. [DOI] [PubMed] [Google Scholar]
- Husum H., Mathé A.A. Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology. 2002;27:756–764. doi: 10.1016/S0893-133X(02)00363-9. [DOI] [PubMed] [Google Scholar]
- Igwe J.C., Jiang X., Paic F., Ma L., Adams D.J., Baldock P.A., Pilbeam C., Kalajzic I. Neuropeptide Y is expressed by osteocytes and can inhibit osteoblastic activity. J. Cell Biochem. 2009;108:621–630. doi: 10.1002/jcb.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G. The central regulation of bone remodeling. Trends Endocrinol. Metab. 2000;11:437–439. doi: 10.1016/s1043-2760(00)00322-2. [DOI] [PubMed] [Google Scholar]
- Kobold D., Grundmann A., Piscaglia F., Eisenbach C., Neubauer K., Steffgen J., Ramadori G., Knittel T. Expression of reelin in hepatic stellate cells and during hepatic tissue repair: a novel marker for the differentiation of HSC from other liver myofibroblasts. J. Hepatol. 2002;36:607–613. doi: 10.1016/s0168-8278(02)00050-8. [DOI] [PubMed] [Google Scholar]
- Lanyon L.E. Osteocytes, strain detection, bone modeling and remodeling. Calcified Tissue Int. 1993;53:102–107. doi: 10.1007/BF01673415. [DOI] [PubMed] [Google Scholar]
- Lussier A.L., Caruncho H.J., Kalynchuk L.E. Repeated exposure to corticosterone, but not restraint, decreases the number of reelin-positive cells in the adult rat hippocampus. Neurosci. Lett. 2009;460:170–174. doi: 10.1016/j.neulet.2009.05.050. [DOI] [PubMed] [Google Scholar]
- Lussier A.L., Romay-Tallón R., Kalynchuk L.E., Caruncho H.J. Reelin as a putative vulnerability factor for depression: Examining the depressogenic effects of repeated corticosterone in heterozygous reeler mice. Neuropharmacology. 2011;60:1064–1074. doi: 10.1016/j.neuropharm.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Marotti G. The osteocyte as a wiring transmission system. J. Musculoskel. Neuron Interact. 2000;1:133–136. [PubMed] [Google Scholar]
- Maurin J.C., Couble M.L., Didier-Bazes M., Brisson C., Magloire H., Bleicher F. Expression and localization of reelin in human odontoblasts. Matrix Biol. 2004;23:277–285. doi: 10.1016/j.matbio.2004.06.005. [DOI] [PubMed] [Google Scholar]
- McKibbin P.E., Cotton S.J., McCarthy H.D., Williams G. The effect of dexamethasone on neuropeptide Y concentrations in specific hypothalamic regions. Life Sci. 1992;51:1301–1307. doi: 10.1016/0024-3205(92)90020-p. [DOI] [PubMed] [Google Scholar]
- Mikami Y., Takahashi T., Kato S., Takagi M. Dexamethasone promotes DMP1 mRNA expression by inhibiting negative regulation of Runx2 in multipotential mesenchymal progenitor, ROB-C26. Cell Biol. Int. 2008;32:239–246. doi: 10.1016/j.cellbi.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Misaki N., Higuchi H., Yamagata K., Miki N. Identification of glucocorticoid responsive elements (GREs) at far upstream of rat NPY gene. Neurochem. Int. 1992;21:185–189. doi: 10.1016/0197-0186(92)90145-h. [DOI] [PubMed] [Google Scholar]
- Necela B.M., Cidlowski J.A. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc. Am. Thoracic Soc. 2004;1:239–246. doi: 10.1513/pats.200402-005MS. [DOI] [PubMed] [Google Scholar]
- Niu S., Yabut O., D’Arcangelo G. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci. 2008;28:10339–10348. doi: 10.1523/JNEUROSCI.1917-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien C.A., Jia D., Plotkin L.I., Bellido T., Powers C.C., Stewart S.A., Manolagas S.C., Weinstein R.S. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- Obata K., Furuno T., Nakanishi M., Togari A. Direct neurite-osteoblastic cell communication, as demonstrated by use of an in vitro co-culture system. FEBS Lett. 2007;581:5917–5922. doi: 10.1016/j.febslet.2007.11.065. [DOI] [PubMed] [Google Scholar]
- Paic F., Igwe J.C., Nori R., Kronenberg M.S., Franceschetti T., Harrington P., Kuo L., Shin D.G., Rowe D.W., Harris S.E. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 2009;45:682–692. doi: 10.1016/j.bone.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachroni K.K., Karatzas D.N., Papavassiliou K.A., Basdra E.K., Papavassiliou A.G. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol. Med. 2009;15:208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Pulido J.S., Sugaya I., Comstock J., Sugaya K. Reelin expression is upregulated following ocular tissue injury. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:889–893. doi: 10.1007/s00417-006-0458-4. [DOI] [PubMed] [Google Scholar]
- Rawlinson S.C.F., McKay I.J., Ghuman M., Wellmann C., Ryan P., Prajaneh S., Zaman G., Hughes F.J., Kingsmill V.J. Adult rat bones maintain distinct regionalized expression of markers associated with their development. PloS One. 2009;4:e8358. doi: 10.1371/journal.pone.0008358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt H.M., Schütz G. Glucocorticoid signalling-multiple variations of a common theme. Mol. Cell. Endocrinol. 1998;146:1–6. doi: 10.1016/s0303-7207(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Rho J., Takami M., Choi Y. Osteoimmunity: interactions of the immune and skeletal systems. Mol. Cells. 2004;17:1–9. [PubMed] [Google Scholar]
- Royaux I., de Rouvroit C.L., D’Arcangelo G., Demirov D., Goffinet A.M. Genomic organization of the mouse reelin gene. Genomics. 1997;46:240–250. doi: 10.1006/geno.1997.4983. [DOI] [PubMed] [Google Scholar]
- Schrauwen I., Ealy M., Huentelman M.J., Thys M., Homer N., Vanderstraeten K., Fransen E., Corneveaux J.J., Craig D.W., Claustres M., et al. A genome-wide analysis identifies genetic variants in the RELN gene associated with otosclerosis. Am. J. Hum. Genet. 2009;84:328–338. doi: 10.1016/j.ajhg.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M.S. The nerves of bone. J. Bone Joint Surg. 1963;45A:522–528. [Google Scholar]
- Shimizu H., Arima H., Watanabe M., Goto M., Banno R., Sato I., Ozaki N., Nagasaki H., Oiso Y. Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via adenosine monophosphate-activated protein kinase signaling in the arcuate nucleus of rats. Endocrinology. 2008;149:4544–4553. doi: 10.1210/en.2008-0229. [DOI] [PubMed] [Google Scholar]
- Smalheiser N.R., Costa E., Guidotti A., Impagnatiello F., Auta J., Lacor P., Kriho V., Pappas G.D. Expression of reelin in adult mammalian blood, liver, pituitary pars intermedia, and adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA. 2000;97:1281–1286. doi: 10.1073/pnas.97.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga S., Goto S., Togari A. Demonstration of direct neurite-osteoclastic cell communication in vitro via the adrenergic receptor. J. Pharmacol. Sci. 2010;112:184–191. doi: 10.1254/jphs.09283fp. [DOI] [PubMed] [Google Scholar]
- Teixeira L., Sousa D.M., Nunes A.F., Sousa M.M., Herzog H., Lamghari M. NPY revealed as a critical modulator of osteoblast function in vitro: new insights into the role of Y1 and Y2 receptors. J. Cell. Biochem. 2009;107:908–916. doi: 10.1002/jcb.22194. [DOI] [PubMed] [Google Scholar]
- Titolo D., Mayer C.M., Dhillon S.S., Cai F., Belsham D.D. Estrogen facilitates both phosphatidylinositol 3-kinase/Akt and ERK1/2 mitogen-activated protein kinase membrane signaling required for long-term neuropeptide Y transcriptional regulation in clonal, immortalized neurons. J. Neurosci. 2008;28:6473–6482. doi: 10.1523/JNEUROSCI.0514-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zande M., Walboomers X.F., Brannvall M., Olalde B., Jurado M.J., ¢̈lava J.I., Jansen J.A. Genetic profiling of osteoblast-like cells cultured on a novel bone reconstructive material, consisting of poly-l-lactide, carbon nanotubes and microhydroxyapatite, in the presence of bone morphogenetic protein-2. Acta Biomater. 2010;6:4352–4360. doi: 10.1016/j.actbio.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Wang J., Akabayashi A., Dourmashkin J., Yu H.J., Alexander J.T., Chae H.J., Leibowitz S.F. Neuropeptide Y in relation to carbohydrate intake, corticosterone and dietary obesity. Brain Res. 1998;802:75–88. doi: 10.1016/s0006-8993(98)00551-4. [DOI] [PubMed] [Google Scholar]
- Weinstein R.S., Jilka R.L., Parfitt A.M., Manolagas S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbroek I., van der Plas A., de Rooij K.E., Klein-Nulend J., Nijweide P.J. Expression of serotonin receptors in bone. J. Biol. Chem. 2001;276:28961–28968. doi: 10.1074/jbc.M101824200. [DOI] [PubMed] [Google Scholar]
- Zengin A., Zhang L., Herzog H., Baldock P.A., Sainsbury A. Neuropeptide Y and sex hormone interactions in humoral and neuronal regulation of bone and fat. Trends Endocrinol. Metab. 2010;21:411–418. doi: 10.1016/j.tem.2010.02.004. [DOI] [PubMed] [Google Scholar]