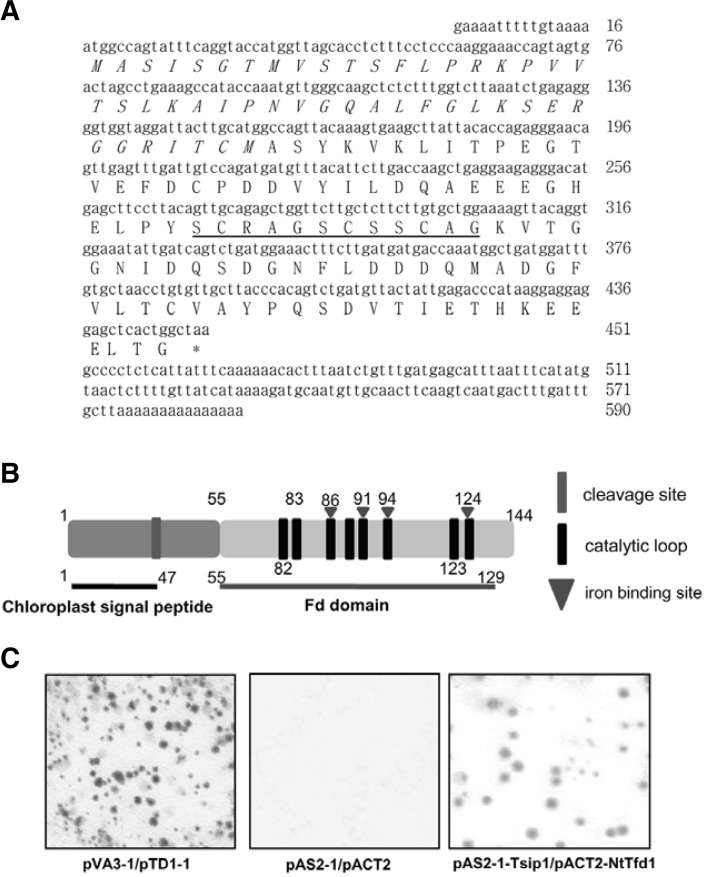
Fig. 1.
Identification of tobacco ferredoxin 1 (NtTfd1) protein as a Tsip1-interacting protein. The nucleotide and the deduced amino acid sequence of the NtTfd1 cDNA clone. The NtTfd1 gene con-tained an open reading frame (ORF) of 435 bp encoding a poly-peptide of 145 amino acids. The chrolopast transit peptide is marked by italic letter and the iron-sulfer cluster domain of Fd, which plays a role in electron transfer, is underlined. (B) Schematic representation of NtTfd1 by bioinformatic tools. Chloroplast target signal peptide sequence and cleavage site were predicted by ChloroP1.1 server (http://www.cbs.dtu.dk/services/ChloroP/). Fd iron binding site and caltalytic loop were predicted by NCBI Conserved Domian Search (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). (C) Activation of the GAL4 operon by the interaction between each designated bait and the putative interacting protein is shown by colony-lift filter assay. The spots indicate β-galactosidase activity on the X-gal substrate. The yeast strain Y187 containing the plasmids pVA3-1 and pTD1-1 were used as a positive control. pAS2-1 and pACT2 empty vectors in yeast cell were used as the negative control. The yeast strain Y187 containing the plasmids pAS2-1-Tsip1 and pACT2-NtTfd1 showed β-galactosidase activity in a colony-lift filter assay.