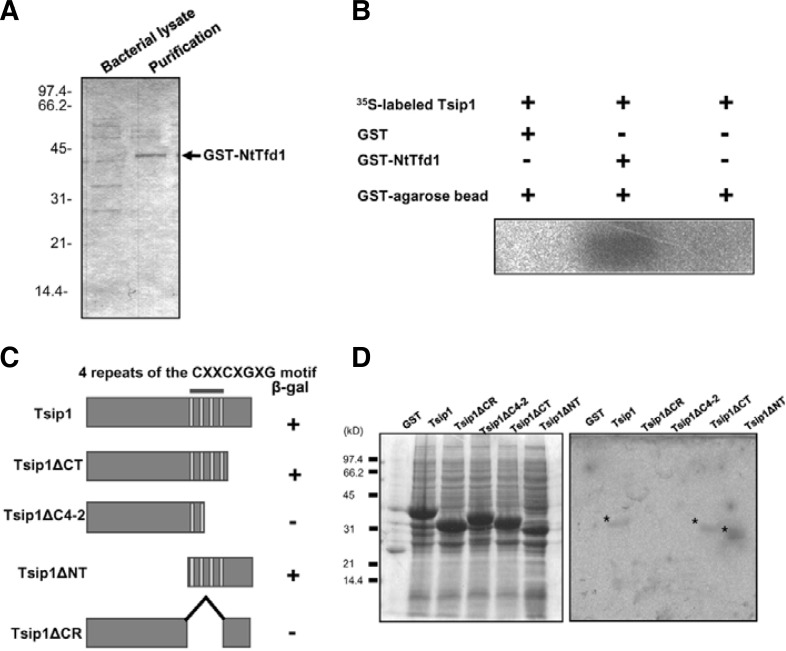
Fig. 2.
Interaction of NtTfd1 with Tsip1 protein in vitro. (A) Affinity purification of GST-NtTfd1 fusion protein. The purified proteins were separated by 12% SDS-PAGE and stained with Coomassie brilliant blue. (B) In vitro binding assay. GST-NtTfd1 protein was incubated with in vitro translated 35S-labeled Tsip1 protein and visualized by autoradiography. (C) Schematic representation of the constructs used in the yeast two-hybrid interation assay. The indicated regions of Tsip1 were cloned into the bait vector pAS2-1 and the Tsip1 bait plasmids were transformed with NtTfd1 into the yeast. β-galactosidase activities are shown from the combination of individual Tsip1 deletion constructs with NtTfd1. The symbol (+) indicates interaction and (−) indicates non-interaction. (D) In vitro binding assay results. Samples of GST-fused Tsip1 and Tsip1 deletion mutants were fractionated into insoluble fractions. Proteins were separated by 12% SDS-PAGE and stained with Comassie brilliant blue (left) or a far-Western blot analysis was performed to test binding of 35S-labeled NtTfd1 probe to the immobilized Tsip1 deletion mutants (right). Asterisks indicate the binding.