Abstract
Defensins, a family of antimicrobial peptides, are one of the first lines of host defense. Human beta-defensins (hBD) such as hBD-2 and -3 have anti-HIV activity. Previous studies have shown that HIV-1 virion can induce the expression of hBD, although the exact components of HIV-1 virion that are responsible for hBD expression have not yet been elucidated. In this study, we examined the effect of HIV-1 Tat on the expression of hBD in B cells. Stimulation of B cells with HIV-1 Tat protein significantly increased the mRNA and protein levels of hBD-2. HIV-1 Tat also induced the activation of a reporter gene for hBD-2 in a dose-dependent manner in B cells. Pretreatment of B cells with a JNK inhibitor suppressed HIV-1 Tat-induced hBD-2 expression. Pretreatment of B cells with AP-1 inhibitors or NF-κB inhibitors led to a decrease in HIV-1 Tat-induced protein and mRNA expression of hBD-2. Taken together, our results indicate that HIV-1 Tat can up-regulate the expression of hBD-2 via JNK-NF-κB/AP-1-dependent pathways in human B cells.
Keywords: AP-1, B cells, beta-defensins, HIV, MAPK, NF-κB, Tat
INTRODUCTION
HIV-1 Tat is one of the viral gene products essential for the expression and replication of the viral genome. HIV-1 Tat protein, released from the infected cells, exerts various biological activities on uninfected cells (Ensoli et al., 1993). Therefore, HIV-1 Tat can affect the generation of innate and acquired immunity during HIV infection. It was previously reported that HIV-1 Tat up-regulates the expression of pro-inflammatory mediators such as cytokines, chemokines, and adhesion molecules (Pu et al., 2003; Song et al., 2011) and enzymes including matrix metalloproteinases (MMPs) (Ju et al., 2009) in various cell types including macrophages. However, little information is available about the expression of antimicrobial factors, including defensins, in response to Tat stimulation in the immune system.
Defensins belong to the family of antimicrobial peptides that serve as the first line of host defense against bacterial and viral infection (Lehrer and Ganz, 2002). Previous studies have demonstrated that a class of defensins was an effective inhibitor of HIV-1 infection in vitro and in vivo (for review, see Cole and Lehrer, 2003). Human beta-defensins (hBD), such as hBD-2, have been shown to exert anti-HIV activity (Quiñones-Mateu et al., 2003; Sun et al., 2005; Weinberg et al., 2006). It has been demonstrated that hBD-2 and 3 expressed in human oral epithelial cells inhibited HIV-1 replication via a direct interaction with virions and through modulation of the CXCR4 coreceptor (Quiñones-Mateu et al., 2003; Weinberg et al., 2006). In addition to exerting direct antiviral effects against HIV-1, defensins have immune-stimulatory activity because they mediate the signals involved in adaptive immune responses (Bowdish et al., 2006). hBD is up-regulated in various cell types in response to microbial infection and pro-inflammatory cytokines (Tsutsumi-Ishii et al., 2000; Wehkamp et al., 2006). Previous studies have shown that HIV-1 virion can induce the expression of hBD, even in the absence of HIV-1 replication (Quiñones-Mateu et al., 2003). However, little is known about the molecular mechanisms by which HIV-1 induces the expression of hBD. In addition, the viral components responsible for hBD expression have not been determined.
The innate immune response occurs rapidly after entry of HIV and collaborates with adaptive immune responses to combat HIV infection. Since conventional approaches using the adaptive immune system failed to achieve protection from HIV infection, new antiviral approaches from the innate immune system could be developed based on host-virus interaction. B cells play a major role in the adaptive immune response by producing specific antibodies against viral infection. However, the contribution of B cells to the innate immune response is largely unknown. Although previous studies showed that B lymphocytes and plasma cells expressed hBD-2 protein (Han et al., 2009; Rahman et al., 2007), little information is available on production of defensins in B cells during HIV infection. Therefore, we sought to determine whether human B cells express hBD-2 upon HIV-1 Tat stimulation.
In this study, we examined the effect of extracellular HIV-1 Tat on the expression of HBD-2 and its underlying action mechanisms in a human B cell line, RPMI 8226. We show for the first time that HIV-1 Tat increases the level of HBD-2 expression via JNK/AP-1/NF-κB- dependent pathways in human B cells.
MATERIALS AND METHODS
Cell culture and reagents
RPMI 8226, a human B cell line, was obtained from the American Type Culture Collection (USA) and was maintained in an RPMI 1640 medium with 10% (v/v) heat-inactivated fetal bovine serum (Han et al., 2009). N-α-p-tosyl-L-lysine chloromethyl ketone hydrochloride (TLCK), BMS 345541, SB203580, and PD98059 were purchased from Sigma (USA). SP600125 was purchased from Calbiochem (USA). Primary antibodies against phospho-IκBα, IκBα, phospho-p65, phosphor-c-jun, c-jun, c-fos, phospho-ERK, ERK, phospho-p38, p38, phospho-JNK and JNK (Cell Signaling Technology, USA) were obtained commercially. HRP-conjugated anti-rabbit and goat antibodies were supplied by Sigma (USA).
Purification of recombinant HIV-1 Tat protein
Recombinant HIV-1 Tat protein was purified under native conditions as described previously (Song et al., 2011). Endotoxin levels for the Tat preparation were below the detection limit (< 0.1 EU/ml) as measured by a Limulus Amoebocyte Lysate assay (BioWhitaker, Walkersville, MD, USA). The integrity and purity of the HIV-1 Tat proteins were assessed by SDS-PAGE followed by Coomassie blue staining. The biological activity of Tat was confirmed by a transactivation assay in HeLa cells transfected with a plasmid containing an HIV long terminal repeat (LTR)-luciferase gene.
Western blot analysis
Cell lysates were prepared by incubating cells in lysis buffer (125 mM Tris-HCl pH 6.8, 2% SDS, 10% v/v glycerol.) at 4°C for 30 min (Lee et al., 2010). Samples of fifty μg protein were fractionated by electrophoresis on a 10% sodium dodecyl sulfate-polyacrylamide gel. The proteins were electrotransferred to a nitrocellulose membrane, which was blocked with 10% dry milk in PBS. The membrane was probed with the indicated antibodies, and the immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham) following manufacturer instruction.
RT-PCR analysis
Total RNA was extracted with a Trizol reagent kit (Invitrogen, USA) according to the manufacturer’s instructions. The RNA (2 μg) was reversibly transcribed with 10,000 U of reverse transcriptase and 0.5 μg/μl oligo-(dT)15 primer (Promega, USA). The cDNA mixture was subjected to the standard PCR reaction for 30 cycles. To analyze the expression of the human defensin family, we used the primers as previously described (Han et al., 2009). The β-actin expression level was used as a control: human β-actin, 5′-GGGTCAGAAGGATTCCTATG-3′ and 5′-CCTTAATGTCACGCACGATTT-3′. PCR was performed in 50 μl of 10 mmol/L Tris-HCl (pH 8.3), 25 mmol/L MgCl2, 10 mmol/L dNTP, 100 U of Taq DNA polymerase, and 0.1 μmol/L of each primer and was terminated by heating at 70°C for 15 min. The PCR products were resolved on a 1% agarose gel and visualized with UV light after ethidium bromide staining.
Measurement of hBD production by ELISA
B cells were stimulated with HIV-1 Tat for the indicated periods, and the levels of hBD in the supernatants were measured by an ELISA kit (Peprotech, USA), according to the manufacturer’s instructions.
Transfection
NF-κB-luc reporter plasmid (Stratagene, USA) and hBD-2 luciferase plasmid (phBD-2-luc) containing regions spanning −253 to +111 of the human hBD-2 promoter were used (Han et al., 2009). Transfection of B cells with phBD-2-luc, pNF-κB-luc or pCMV-β-galactosidase construct was performed using using FuGENE 6 (Roche, USA) transfection reagent following the manufacturer’s instruction. After 24 h, the cells were harvested, and luciferase and beta-galactosidase activities were measured. The luciferase activity of each sample was normalized to the beta-galactosidase activity to calculate the relative luciferase activity, and the results were expressed as fold transactivation.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts of cells were prepared and analyzed for NF-κB binding activity by EMSA as described previously (Lee et al., 2010). An NF-κB or AP-1 consensus oligonucleotide (Promega) was used in the EMSA. The complementary oligonucleotide was annealed and end-labeled with [γ-32P]ATP using T4 polynucleotide kinase. EMSA was performed in a total volume of 20 μl at 4°C. Five micrograms of nuclear extracts were equilibrated for 15 min in binding buffer (10 mM Tris-HCl, pH 8.0, 75 mM KCl, 2.5 mM MgCl2, 0.1 mM EDTA, 10% glycerol, 0.25 mM dithiothreitol) and 1 μg of poly dI/dC. 32P-labeled oligonucleotide probe (20,000 cpm) was then added and the reaction was incubated on ice for an additional 20 min. Bound and free DNA were then resolved by electrophoresis on a 6% native polyacrylamide gel in TBE buffer (89 mM Tris-HCl, 89 mM boric acid, and 2 mM EDTA).
Statistical analysis
The results were expressed as the mean ± SEM from at least three independent experiments. The values were evaluated via one-way ANOVA, followed by Duncan’s multiple range tests using GraphPad Prism 4.0 software (GraphPad Software, Inc., USA). Differences were considered to be significant at p < 0.001.
RESULTS
Induction of hBD expression by HIV-1 Tat in human B cells
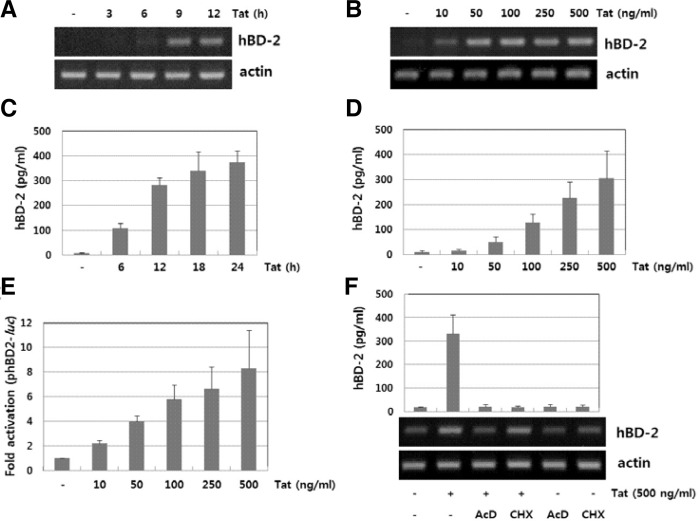
It has been demonstrated that human oral epithelial cells expressed hBD-2 and 3 mRNA upon stimulation with HIV-1 virions (Quiñones-Mateu et al., 2003). We hypothesized that besides the HIV-1 virion, viral proteins such as HIV-1 Tat may contribute to up-regulate expression of hBD. We previously reported that human B cells could express hBD-2 upon stimulation with LPS or CpG-DNA (Han et al., 2009). We first analyzed the expression pattern of the defensin family in the human B cell line RPMI 8226 after HIV-1 Tat stimulation by RT-PCR. HIV-1 Tat was able to induce the expression of hBD-2 but not that of other defensins (data not shown). To examine the expression profiles of hBD-2 at the transcriptional level, cells were exposed to varying doses of HIV-1 Tat (10–500 ng/ml) for the indicated times and then the induction of hBD-2 mRNA expression was analyzed by RT-PCR in HIV-1 Tat-stimulated RPMI 8226 cells. As shown in Figs. 1A and 1B, HIV-1 Tat increased the levels of hBD-2 mRNA in a time- and dose-dependent manner in RPMI 8226 cells. To assess the production of hBD-2 in B cells, cells were exposed to varying doses of HIV-1 Tat (10–500 ng/ml) for the indicated times. We analyzed the levels of hBD-2 production in the culture supernatants by ELISA. Stimulation with HIV-1 Tat resulted in the production of hBD-2 in a time- and dose-dependent manner (Figs. 1C and 1D). We further evaluated the effect of HIV-1 Tat on hBD-2 promoter activity. RPMI 8226 cells transfected with a phBD-2 promoter-luciferase construct were stimulated with HIV-1 Tat and the luciferase activity was measured (Fig. 1E). HIV-1 Tat increased phBD-2 promoter activity in a dose-dependent manner. In addition, treatment with actinomycin D, an inhibitor of mRNA synthesis, and cycloheximide, an inhibitor of protein synthesis, markedly decreased HIV-1 Tat-induced hBD-2 expression (Fig. 1F), indicating that de novo mRNA synthesis is required for HIV-1 Tat-induced hBD-2 gene expression. These results imply that upon stimulation with HIV-1 Tat, human B cells can induce expression and release of the hBD-2 in the B cells.
Fig. 1.
Expression of hBD-2 in HIV-1 Tat-stimulated cells. RPMI 8226 cells were treated with HIV-1 Tat (500 ng/ml) for the indicated time or at various concentrations of Tat for 12 h. The total RNA and protein in the culture supernatants were analyzed by RT-PCR (A, B) and ELISA (C, D), respectively. (E) Activation of hBD-2 promoter by HIV-1 Tat. The transfected RPMI 8226 cells with phBD-2-Luc were exposed to HIV-1 Tat for 24 h and the luciferase activity was measured. (F) Effect of metabolic inhibitors on HIV-1 Tat-induced hBD-2 expression. RPMI 8226 cells pretreated with actinomycin D (10 μg/ml) or cyclohexamide (10 μM) for 1 h were exposed to HIV-1 Tat. Expression of hBD mRNA and protein was analyzed by RT-PCR and ELISA, respectively.
HIV-1 Tat induced JNK activation that is required for hBD-2 expression in B cells
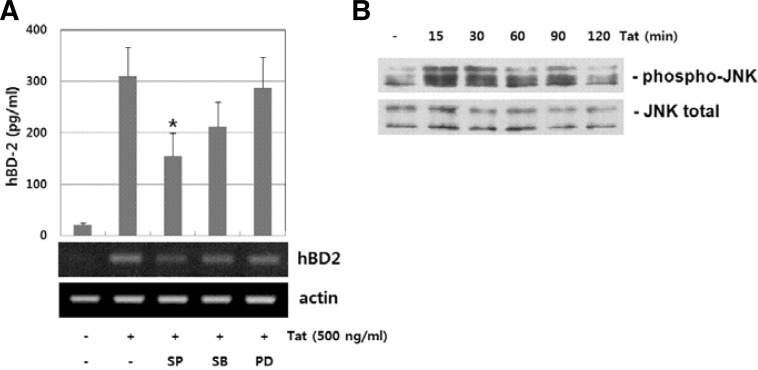
Previous studies have indicated that the activity of MAP kinases such as p38, JNK and ERK protein kinase is involved in hBD-2 expression upon various stimuli (Jang et al., 2004; Méndez-Samperio et al., 2007; Yoon et al., 2010). To further analyze the effect of the MAPK superfamily pathway on HIV-1 Tat-mediated hBD-2 production, we measured HIV-1 Tat-induced hBD-2 production in the presence of pathway-specific inhibitors. As shown in Fig. 2A, JNK inhibitor SP600125 significantly suppressed HIV-1 Tat-induced hBD-2 protein production, while MEK1/2 inhibitor PD98059 and p38 MAP kinase inhibitor SB203580 have a minimal effect. HIV-1 Tat induced phosphorylation of JNK protein kinase in a time-dependent manner (Fig. 2B). These results indicate that JNK activity is required for HIV-1 Tat-induced hBD-2 expression.
Fig. 2.
JNK activation in HIV-1 Tat-induced hBD-2 expression. (A) Dependency of MAPK activation in HIV-1 Tat-induced hBD-2 expression. RPMI 8226 cells were treated with HIV-1 Tat in the absence or presence of MAPK inhibitors (20 μM SP600125, 20 μM SB203580 and 20 μM PD98059). Expression of hBD mRNA and protein was analyzed by RT-PCR and ELISA, respectively. The results are means ± SD of three separate experiments. An asterisk indicates statistical significance at P < 0.001 as compared with cells treated with HIV-1 Tat alone. (B) Analysis of JNK activation in HIV-1 Tat-stimulated cells. Cells were treated with Tat (500 ng/ml) for the indicated time. Whole cell lysates were analyzed by Western blot analysis using specific antibody for phosphor-JNK. Equal lane loading was confirmed by detecting blot for total JNK.
AP-1 activity is involved in HIV-1 Tat-induced hBD-2 expression human B cells
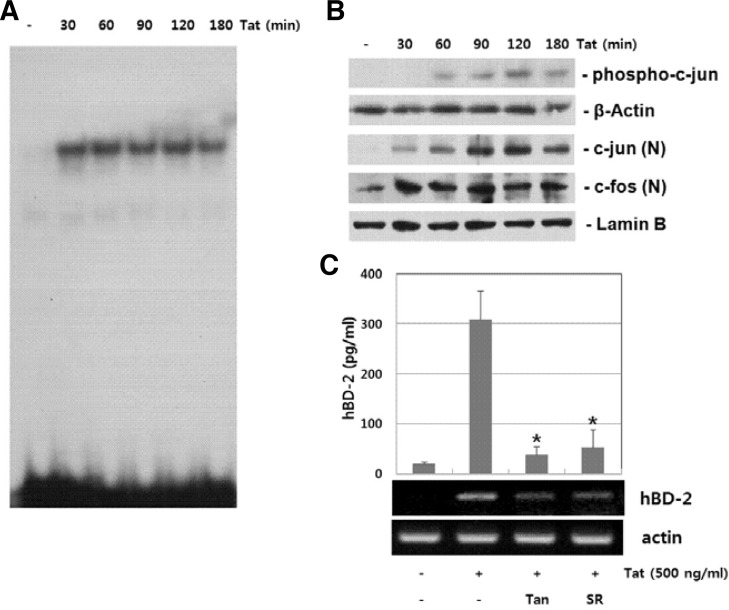
The role of JNK activity in HIV-1 Tat-induced hBD-2 expression suggests that AP-1 activity contributes to HIV-1 Tat-induced hBD-2 expression. We examined the involvement of AP-1 signaling cascades in HIV-1 Tat-induced hBD expression. B cells were stimulated with HIV-1 Tat and then AP-1 activation was analyzed by EMSA. DNA binding activity of AP-1 increased as early as 30 min and was maintained up to 3 h in HIV-1 Tat-stimulated B cells (Fig. 3A). Next, we examined HIV-1 Tat-induced signal cascade of AP-1 activation, such as c-jun phosphorylation and translocation of c-jun/c-fos, by Western blot analysis. Stimulation with HIV-1 Tat resulted in the phosphorylation of c-jun as well as translocation of c-jun/c-fos (Fig. 3B). To explore the functional relationship between AP-1 activation and hBD-2 expression, we used pharmacological inhibitors of AP-1. As shown in Fig. 3C, AP-1 inhibitors, Tanshinone IIA and SR11302, significantly suppressed HIV-1 Tat-induced hBD-2 expression. These results indicate that AP-1 signaling cascades are involved in HIV-1 Tat-induced hBD-2 expression.
Fig. 3.
AP-1 dependent induction of hBD-2 expression in HIV-1 Tat-stimulated cells. (A) Anaysis of AP-1 DNA binding activity. Nuclear extracts prepared from HIV-1 Tat-stimulated cells were analyzed for DNA binding activity of AP-1 by EMSA. (B) Activation of c-jun/c-fos in HIV-1 Tat-stimulated cells. Whole cell lysates or nuclear extracts from RPMI 8226 cells treated with HIV-1 Tat (500 ng/ml) were analyzed by Western blot analysis. (C) Effect of AP-1 inhibitors on HIV-1 Tat-induced hBD-2 expression. RPMI 8226 cells were treated with AP-1 inhibitors (20 μM Tanshinone IIA and 10 μM SR11302) for 1 h, and then exposed to HIV-1 Tat (500 ng/ml). Expression of hBD mRNA and protein was analyzed by RT-PCR and ELISA, respectively. The results are means ± SD of three separate experiments. Asterisk indicates statistical significance at P < 0.001 as compared with cells treated with HIV-1 Tat alone.
NF-κB is responsible for HIV-1 Tat-induced hBD-2 expression in human B cells
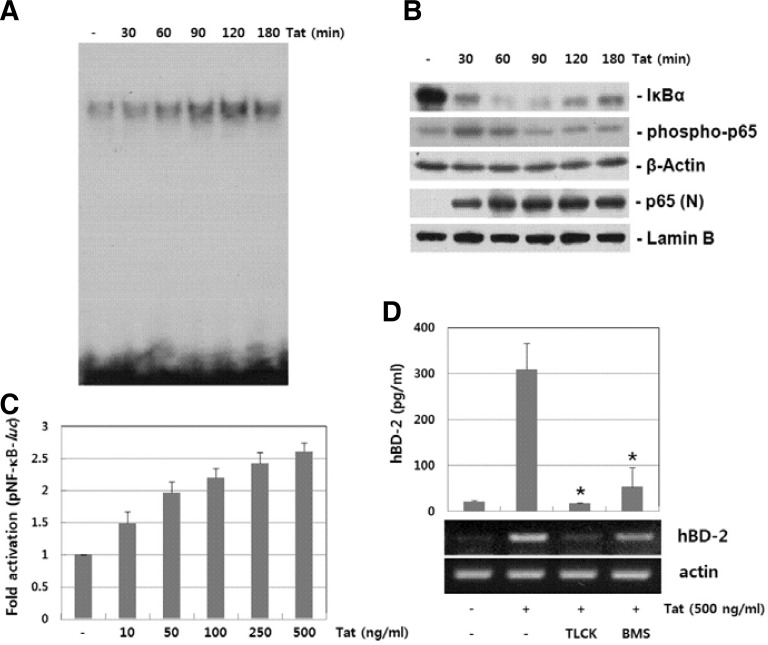
NF-κB signaling pathway is another pathway involved in hBD-2 (Tsutsumi-Ishii et al., 2000; Wehkamp et al., 2006). We examined HIV-1 Tat-induced NF-κB signaling cascades. Nuclear extracts from HIV-1 Tat-stimulated B cells were analyzed by using EMSA. As shown in Fig. 4A, stimulation with HIV-1 Tat increased the DNA binding activity of NF-κB in a time-dependent manner. We further examined whether HIV-1 induces the phosphorylation and nuclear translocation of p65 (a subunit of NF-κB) in B cells. HIV-1 Tat induced the phosphorylation as well as nuclear localization of p65 (Fig. 4B). In addition, HIV-1 Tat induced degradation of IκBα. We next evaluated the effect of HIV-1 Tat on NF-κB promoter activity. B cells transfected with an NF-κB promoter-luciferase construct were stimulated with HIV-1 Tat and luciferase activity was measured. As shown in Fig. 4C, HIV-1 Tat increased NF-κB promoter activity in a dose-dependent manner. As shown in Fig. 4D, NF-κB inhibitors, TLCK and BMS345541, significantly suppressed HIV-1 Tat-induced hBD-2 expression. These results suggest that the signaling pathways that lead to activation of NF-κB play a role in HIV-1 Tat-induced hBD-2 expression in B cells.
Fig. 4.
Involvement of NF-κB activity in HIV-1 Tat-induced hBD-2 expression. (A) Anaysis of NF-κB DNA binding activity. Nuclear extracts prepared from HIV-1 Tat-stimulated cells were analyzed for DNA binding activity of NF-κB by EMSA. (B) IκBα degradation and p65 activation in HIV-1 Tat-stimulated cells. Whole cell lysates or nuclear extracts from RPMI 8226 cells treated with HIV-1 Tat (500 ng/ml) were analyzed by Western blot analysis. (C) Effect of HIV-1 Tat on NF-κB promoter activity. RPMI 8226 cells transiently transfected with a NF-κB promoter-luciferase constract and a β-galactosidase construct were stimulated with HIV-1 for 24 h, and the luciferase activity was determined. (D) Effect of NF-κB inhibitors on HIV-1 Tat-induced hBD-2 expression. RPMI 8226 cells pretreated with NF-κB inhibitors (50 μM TLCK and 5 μM BMS345541) for 1 h were stimulated with HIV-1 Tat (500 ng/ml) for 12 h (for mRNA) or 24 h (for protein). Expression of hBD mRNA and protein was analyzed by RT-PCR and ELISA, respectively. The results are means ± SD of three separate experiments. An asterisk indicates statistical significance at P < 0.001 as compared with cells treated with HIV-1 Tat alone.
DISCUSSION
Innate immune responses are first lines of host defense that function to protect the host upon HIV infection. Antimicrobial peptides such as defensins serve to combat bacterial and viral infection. Human beta-defensins (hBD) such as hBD-2 have been shown to exert anti-HIV activity (Quiñones-Mateu et al., 2003; Sun et al., 2005; Weinberg et al., 2006). hBD is up-regulated in various cell types in response to bacterial infection and pro-inflammatory cytokines (Tsutsumi-Ishii et al., 2000; Wehkamp et al., 2006). Previous studies have shown that HIV-1 virion can induce the expression of hBD, even in the absence of HIV-1 replication (Quiñones-Mateu et al., 2003). However, the viral components responsible for hBD expression have not been determined. In this study, we show that HIV-1 Tat increases the level of hBD-2 production via JNK/AP-1/NF-κB-dependent mechanisms in B lymphocytes.
A variety of defensins, such as alpha-defensin, beta-defensin, and theta-defensin, have been shown to exert anti-HIV activity by multiple distinct mechanisms. Alpha-defensin-1 can inhibit HIV-1 replication via blockage of PKC signaling and inhibition of binding of gp120 and CD4 in CD4+ T cells (Chang et al., 2005; Furci et al., 2007). Beta-defensins such as hBD-2 have been shown to inhibit HIV-1 replication via direct interaction with virions and through down-modulation of the CXCR4 coreceptor (Quiñones-Mateu et al., 2003; Sun et al., 2005; Weinberg et al., 2006). Theta-defensins prevented HIV-1 entry by binding gp41 and blocking 6-helix bundle formation (Gallo et al., 2006). It was reported that HIV-1 can induce hBD-2 expression in human oral epithelial cells (Quiñones-Mateu et al., 2003), suggesting an important roles of hBD-2 in controlling mucosal infection (Shin and Choi, 2010). Up-regulation of hBD-2 expression by HIV-1 Tat in B cells may lead to the suppression of HIV replication. HIV-1 Tat is one of target proteins for the immune responses and activates various cells such as lymphocytes, macrophages, and astrocytes to express a variety of genes involved in immune and inflammatory responses (Herbein et al., 2010; Huigen et al., 2004; Song et al., 2011). It was shown that HIV-1 Tat alone can activate B cells to up-regulate Fas expression (Huang et al., 1997). Based on the expression pattern of the defensin family in the human B cell line RPMI 8226, HIV-1 Tat was able to induce hBD-2 expression (Fig. 1) but not that of the other defensins (data not shown).
The mitogen-activated protein kinases (MAPKs) such as p38, JNK and ERK protein kinase are involved in the signaling cascades leading to hBD-2 expression upon various stimuli (Jang et al., 2004; Méndez-Samperio et al., 2007; Yoon et al., 2010). We observed that JNK inhibitor, but not ERK and p38 MAPK inhibitors, suppressed HIV-1 Tat-induced mRNA expression of hBD-2 as well as the production of hBD-2 (Fig. 2A), suggesting that HIV-1 Tat-induced hBD-2 expression is mediated by JNK activity in B cells. As shown in Fig. 2B, HIV-1 Tat can up-regulate JNK activity in B cells. The involvement of JNK activity in HIV-1 Tat-induced hBD-2 expression suggests that AP-1 activity contributes to HIV-1 Tat-induced hBD-2 expression. Since the hBD-2 promoter contains AP-1 binding sites, we evaluated the contribution of AP-1 pathway in HIV-1 Tat-induced hBD-2 expression. We showed that HIV-1 Tat increased AP-1 promoter activity that was suppressed by AP-1 inhibitors Tan and SR (Fig. 3A). In addition, as shown in Fig. 3B, c-fos and c-jun were activated in HIV-1 Tat-stimulated B cells. Besides AP-1, NF-κB is one of the transcriptional factors involved in hBD-2 expression. We performed experiments to analyze the activation of NF-κB and its signaling cascades. NF-κB inhibitors significantly suppressed HIV-1 Tat-induced hBD-2 production (Fig. 2), suggesting that an NF-κB-dependent pathway contributes to HIV-1 Tat-mediated up-regulation of hBD-2 expression and production in B cells.
Previous studies suggest that a high level of hBD-2 production during HIV infection may be associated with resistance to HIV infection (Zapata et al., 2008) and less rapid disease progression in HIV infection. Although hBD-2 and hBD-3 were shown to have inhibitory effect on HIV replication, some of defensins like hBD-5 and hBD-6 enhance HIV-1 infectivity by promoting viral attachment (Rapista et al., 2011). In most cases, the innate immune response would be partially effective in controlling HIV infection.
Along with defensins, other host defense proteins may contribute to the antiviral activities upon HIV infection (reviewed in Lever and Lever 2011): (1) TRIM5alpha was identified as a cellular factor that restricts HIV-1 infection by blocking early and late phases of the HIV life cycle (Greene et al., 2008; Stremlau et al., 2004). (2) APOBEC3, a member of a larger family of cytidine deaminases, was identified to be active against HIV-1 by interacting with Gag and viral RNA (Sheehy et al., 2002). The HIV-1 protein Vif is known to block APOBEC3 via direct binding to APOBEC3 and subsequent degradation by the 26S proteosome (reviewed in Greene et al., 2008). (3) tetherin, a type II membrane glycoprotein, is one of the antiviral factors that inhibit the release of HIV particles. The HIV-1 protein Vpu interacts with tetherin, leading to downregulation of tetherin from the plasma membrane and its subsequent lysosomal degradation (reviewed in Martin-Serrano and Neil, 2011). (4) Finally, SAMHD1 was recently identified to inhibit HIV-1 infection in human dendritic cells and myeloid cells (Hrecka et al., 2011; Laguette et al., 2011). Among viral proteins, Vpx was shown to interact with SAMHD1 and induce proteasomal degradation of SAMHD1, resulting in relieving the inhibition of HIV-1 infection (Hrecka et al., 2011; Laguette et al., 2011). The balance between the host antiviral response and the counteraction of virus may affect the pathogenesis and spread of HIV-1. Since currently approved anti-HIV agents control HIV infection with limited success, and show drug resistance and various adverse effects, the development of new anti-HIV agents is still required (Greene et al., 2008). Better understanding of host defense proteins exerting inhibitory effect on HIV infection will provide potential target for anti-HIV drugs. In addition, future experiments are required to examine whether HIV-1 Tat affects expression of other host defense proteins such as APOBEC3, tetherin and SAMHD1.
In conclusion, we provide evidence that HIV-1 Tat is capable of stimulating hBD-2 expression via JNK/AP-1/NF-κB dependent pathways in human B cells. An elucidation of the mechanism by which HIV-1 Tat induces hBD-2 production in the B cells may help to understand the role of innate immunity during HIV infection.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government [Ministry of Education, Science, and Technology (MEST)] (2009-0084683). This work was also supported by the Priority Research Centers Program through the NRF funded by the Korea government (MEST) (2009-0093812).
REFERENCES
- Bowdish D.M., Davidson D.J., Hancock R.E. Immunomodulatory properties of defensins and cathelicidins. Curr. Top Microbiol. Immunol. 2006;306:27–66. doi: 10.1007/3-540-29916-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.L., Vargas J., Jr., DelPortillo A., Klotman M.E. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. J. Clin. Invest. 2005;115:765–773. doi: 10.1172/JCI200521948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A.M., Lehrer R.I. Minidefensins: antimicrobial peptides with activity against HIV-1. Curr. Pharm. Des. 2003;9:1463–1473. doi: 10.2174/1381612033454667. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Buonaguro L., Barillari G., Fiorelli V., Gendelman R., Morgan R.A., Wingfield P., Gallo R.C. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furci L., Sironi F., Tolazzi M., Vassena L., Lusso P. Alpha-defensins block the early steps of HIV-1 infection: interference with the binding of gp120 to CD4. Blood. 2007;109:2928–2935. doi: 10.1182/blood-2006-05-024489. [DOI] [PubMed] [Google Scholar]
- Gallo S.A., Wang W., Rawat S.S., Jung G., Waring A.J., Cole A.M., Lu H., Yan X., Daly N.L., Craik D.J., et al. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J. Biol. Chem. 2006;281:18787–18792. doi: 10.1074/jbc.M602422200. [DOI] [PubMed] [Google Scholar]
- Greene W.C., Debyser Z., Ikeda Y., Freed E.O., Stephens E., Yonemoto W., Buckheit R.W., Esté J.A., Cihlar T. Novel targets for HIV therapy. Antiviral Res. 2008;80:251–265. doi: 10.1016/j.antiviral.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Han S.H., Kim Y.E., Park J.A., Park J.B., Kim Y.S., Lee Y., Choi I.G., Kwon H.J. Expression of human beta-defensin-2 gene induced by CpG-DNA in human B cells. Biochem. Biophys. Res. Commun. 2009;389:443–448. doi: 10.1016/j.bbrc.2009.08.162. [DOI] [PubMed] [Google Scholar]
- Herbein G., Gras G., Khan K.A., Abbas W. Macrophage signaling in HIV-1 infection. Retrovirology. 2010;7:34. doi: 10.1186/1742-4690-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrecka K., Hao C., Gierszewska M., Swanson S.K., Kesik-Brodacka M., Srivastava S., Florens L., Washburn M.P., Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Li C.J., Pardee A.B. Human immunodeficiency virus type 1 TAT protein activates B lymphocytes. Biochem. Biophys. Res. Commun. 1997;237:461–464. doi: 10.1006/bbrc.1997.7162. [DOI] [PubMed] [Google Scholar]
- Huigen M.C., Kamp W., Nottet H.S. Multiple effects of HIV-1 trans-activator protein on the pathogenesis of HIV-1 infection. Eur. J. Clin. Invest. 2004;34:57–66. doi: 10.1111/j.1365-2362.2004.01282.x. [DOI] [PubMed] [Google Scholar]
- Jang B.C., Lim K.J., Paik J.H., Kwon Y.K., Shin S.W., Kim S.C., Jung T.Y., Kwon T.K., Cho J.W., Baek W.K., et al. Upregulation of human beta-defensin 2 by interleukin-1beta in A549 cells: involvement of PI3K, PKC, p38 MAPK, JNK, and NF-kappaB. Biochem. Biophys. Res. Commun. 2004;320:1026–1033. doi: 10.1016/j.bbrc.2004.06.049. [DOI] [PubMed] [Google Scholar]
- Ju S.M., Song H.Y., Lee J.A., Lee S.J., Choi S.Y., Park J. Extracellular HIV-1 Tat up-regulates expression of matrix metalloproteinase-9 via a MAPK-NF-kappaB dependent pathway in human astrocytes. Exp. Mol. Med. 2009;41:86–93. doi: 10.3858/emm.2009.41.2.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguette N., Sobhian B., Casartelli N., Ringeard M., Chable-Bessia C., Ségéral E., Yatim A., Emiliani S., Schwartz O., Benkirane M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R.I., Ganz T. Defensins of vertebrate animals. Curr. Opin. Immunol. 2002;14:96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]
- Lee J.A., Song H.Y., Ju S.M., Lee S.J., Seo W.Y., Sin D.H., Goh A.R., Choi S.Y., Park J. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 by cell-permeable superoxide dismutase in lipopolysaccharide-stimulated BV-2 microglial cells. Mol. Cells. 2010;29:245–250. doi: 10.1007/s10059-010-0031-1. [DOI] [PubMed] [Google Scholar]
- Lever R.A., Lever A.M. Intracellular defenses against HIV, viral evasion and novel therapeutic approaches. J. Formos. Med. Assoc. 2011;110:350–362. doi: 10.1016/S0929-6646(11)60053-3. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J., Neil S.J. Host factors involved in retroviral budding and release. Nat. Rev. Microbiol. 2011;9:519–531. doi: 10.1038/nrmicro2596. [DOI] [PubMed] [Google Scholar]
- Méndez-Samperio P., Alba L., Trejo A. Mycobacterium bovis-mediated induction of human beta-defensin-2 in epithelial cells is controlled by intracellular calcium and p38-MAPK. J. Infect. 2007;54:469–474. doi: 10.1016/j.jinf.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Pu H., Tian J., Flora G., Lee Y.W., Nath A., Hennig B., Toborek M. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol. Cell. Neurosci. 2003;24:224–237. doi: 10.1016/s1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Quiñones-Mateu M.E., Lederman M.M., Feng Z., Chakraborty B., Weber J., Rangel H.R., Marotta M.L., Mirza M., Jiang B., Kiser P., et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- Rahman A., Fahlgren A., Sitohy B., Baranov V., Zirakzadeh A., Hammarström S., Danielsson A., Hammarström M.L. Beta-defensin production by human colonic plasma cells: a new look at plasma cells in ulcerative colitis. Inflamm. Bowel Dis. 2007;13:847–855. doi: 10.1002/ibd.20141. [DOI] [PubMed] [Google Scholar]
- Rapista A., Ding J., Benito B., Lo Y.T., Neiditch M.B., Lu W., Chang T.L. Human defensins 5 and 6 enhance HIV-1 infectivity through promoting HIV attachment. Retrovirology. 2011;8:45. doi: 10.1186/1742-4690-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy A.M., Gaddis N.C., Choi J.D., Malim M.H. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Shin J.E., Choi Y. Treponema denticola suppresses expression of human beta-defensin-2 in gingival epithelial cells through inhibition of TNFalpha production and TLR2 activation. Mol. Cells. 2010;29:407–412. doi: 10.1007/s10059-010-0048-5. [DOI] [PubMed] [Google Scholar]
- Song H.Y., Ju S.M., Seo W.Y., Goh A.R., Lee J.K., Bae Y.S., Choi S.Y., Park J. Nox2-based NADPH oxidase mediates HIV-1 Tat-induced up-regulation of VCAM-1/ICAM-1 and subsequent monocyte adhesion in human astrocytes. Free Radic. Biol. Med. 2011;50:576–584. doi: 10.1016/j.freeradbiomed.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Stremlau M., Owens C.M., Perron M.J., Kiessling M., Autissier P., Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Sun L., Finnegan C.M., Kish-Catalone T., Blumenthal R., Garzino-Demo P., La Terra Maggiore G.M., Berrone S., Kleinman C., Wu Z., Abdelwahab S., et al. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J. Virol. 2005;79:14318–14329. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi-Ishii Y., Nagaoka I. NF-kappaB-mediated transcriptional regulation of human beta-defensin-2 gene following lipopolysaccharide stimulation. J. Leukoc. Biol. 2002;71:154–162. [PubMed] [Google Scholar]
- Wehkamp K., Schwichtenberg L., Schröder J.M., Harder J. Pseudomonas aeruginosa- and IL-1beta-mediated induction of human beta-defensin-2 in keratinocytes is controlled by NF-kappaB and AP-1. J. Invest. Dermatol. 2006;126:121–127. doi: 10.1038/sj.jid.5700020. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Quiñones-Mateu M.E., Lederman M.M. Role of human beta-defensins in HIV infection. Adv. Dent. Res. 2006;19:42–48. doi: 10.1177/154407370601900109. [DOI] [PubMed] [Google Scholar]
- Yoon Y.M., Lee J.Y., Yoo D., Sim Y.S., Kim Y.J., Oh Y.K., Kang J.S., Kim S., Kim J.S., Kim J.M. Bacteroides fragilis enterotoxin induces human beta-defensin-2 expression in intestinal epithelial cells via a mitogen-activated protein kinase/I kappaB kinase/NF-kappaB-dependent pathway. Infect. Immun. 2010;78:2024–2033. doi: 10.1128/IAI.00118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata W., Rodriguez B., Weber J., Estrada H., Quiñones-Mateu M.E., Zimermman P.A., Lederman M.M., Rugeles M.T. Increased levels of human beta-defensins mRNA in sexually HIV-1 exposed but uninfected individuals. Curr. HIV Res. 2008;6:531–538. doi: 10.2174/157016208786501463. [DOI] [PMC free article] [PubMed] [Google Scholar]