Abstract
The hematopoietic cell malignancy is one of the most prevalent type of cancer and the disease has multiple pathologic molecular signatures. Research on the origin of hematopoietic cancer stem cells and the mode of subsequent maintenance and differentiation needs robust animal models that can reproduce the transformation and differentiation event in vivo. Here, we show that co-transduction of MYC and PIM2 proto-oncogenes into mouse bone marrow cells readily establishes permanent cell lines that can induce lethal myeloid sarcoma in vivo. Unlike the previous doubly transgenic mouse model in which co-expression of MYC and PIM2 transgenes exclusively induced B cell lymphoma, we were able to show that the same combination of genes can also transform primary bone marrow myeloid cells in vitro resulting in permanent cell lines which induce myeloid sarcoma upon in vivo transplantation. By inducing cancerous transformation of fresh bone marrow cells in a controlled environment, the model we established will be useful for detailed study of the molecular events involved in initial transformation process of primary myeloid bone marrow cells and provides a model that can give insight to the molecular pathologic characteristics of human myeloid sarcoma, a rare presentation of solid tumors of undifferentiated myeloid blast cells associated with various types of myeloid leukemia.
Keywords: animal model, MYC, myeloid sarcoma, PIM2
INTRODUCTION
Animal model systems of leukemia and lymphoma are useful in elucidating the molecular and cellular characteristics of human leukemia and are also invaluable in identifying cellular origins of hematologic malignancies. In fact, the lineage relationship of leukemia stem cells was described first in a xenograft animal model system of human leukemia (Dick et al., 1997). Model systems are also useful in developing and testing various therapeutic reagents for leukemia treatment as well as discovering various proto-oncogenes responsible for leukemia induction (Cuenco et al., 2000; Lavau et al., 2000; Yilmaz et al., 2006; Zhang et al., 2006). Among several different categories of animal models, xenograft models and genetically engineered models are both valuable for studying various aspects of human hematopoietic cancer. For example, a xenograft model which utilizes non-obese diabetic mice with severe combined immunodeficiency disease (NOD/SCID) to transplant and maintain of human leukemic cells (Cashman et al., 1997; Lapidot et al., 1997) has been used to develop several new treatment modalities (Guzman et al., 2002; Iversen et al., 2002; Wierenga et al., 2003). The recent development of intra-bone marrow injection technique has dramatically increased engraftment rates for human leukemic cells (Wang et al., 2003). The genetically engineered model includes various transgenic and ‘knock-in’ models. Transgenic mouse models have been particularly useful in elucidating the biological role of various fusion genes created by chromosomal translocations and inversions, such as PML/RARα (Grisolano et al., 1997), PLZF/RARα (Cheng et al., 1999), and AML1/ETO (Yuan et al., 2001). In the classical transgenic mouse model, transgenes are overexpressed in most tissues. However, expression cassettes employing regulatory units of MRP8 (Brown et al., 1997) and human cathepsin-G (Grisolano et al., 1994) genes have been used to achieve myeloid-specific expression of transgenes in some studies.
Although these animal models have been invaluable in studying various aspects of leukemia, detailed study of early events in leukemic transformation has been hindered by the uncontrolled nature of disease onset and progression. For example, the PIM2 / MYC bi-transgenic mouse model of leukemia develops extensive B cell leukemia and lymphoma within 3 weeks after birth (Allen et al., 1997), but it is not possible to study initial leukemic changes that occur in the bone marrow or embryonic hematopoietic stem/progenitor cells in this system.
Transplantation of retrovirally transduced cells is another useful genetically engineered model system for studying leukemia. For example, the cooperation of TEL/PDGFR and AML1/ETO (Grisolano et al., 2003), HOXA9 and MEIS1 (Kroon et al., 1998; Lawrence et al., 1999), HOXA9 and E2A/PBX1 (Thorsteinsdottir et al., 1999) in leukemia induction have been demonstrated by retroviral co-transduction and transplantation studies. In these systems, the transduced bone marrow progenitor cells are transplanted into γ-radiation-conditioned mice immediately after transduction thus precluding detailed study of early events of transformation.
In this study, we co-transduced PIM2 and MYC proto-oncogenes to transform mouse bone marrow progenitor cells in vitro and maintained the transformed cells in culture to establish malignant cell lines with in vivo leukemogenic potential. With this system, the whole transformation process proceeds in culture allowing detailed study of initial transformation processes in primary bone marrow progenitor cells.
Previously, PIM2/MYC co-expression has been shown to induce B cell lymphoma in a bi-transgenic animal model system in which MYC was expressed under the control of Eμ enhancer (Allen et al., 1997). Here we show that the same combination of genes shows potent synergy in transforming primary mouse bone marrow myeloid progenitors in vitro, resulting in permanent cell lines. This model system will allow investigation of early events in the malignant transformation of myeloid cells. Furthermore, our established cell lines transformed with PIM2/MYC reliably induce lethal myeloid sarcomas upon in vivo transplantation, with negligible levels of circulating leukemic cells in blood or spleen. Therefore, our animal model will also provide a unique opportunity to explore critical parameters in development of myeloid sarcomas associated with myelodysplastic syndromes and hematopoietic malignancies.
MATERIALS AND METHODS
Construction of the plasmid and retrovirus production
Retroviral vectors were constructed that could express both PIM2 (MGC clone; 8925 Thermo) and green fluorescence protein (GFP) by inserting internal r ibosomal entry site (IRES) in pMSCV (Clontech, USA). Retroviral vector expressing MYC (MGC clone; 5183, Thermo) was constructed in same way as PIM2 except the truncated nerve growth factor receptor (tNGFR) (Robbins et al., 1997) was used as a marker instead of GFP. Retroviruses were harvested from 293T cells 48 h after co-transfection of retroviral vector plasmid, pMD gag/pol, and pMD. VSV-G as described previously (Ory et al., 1996).
Retroviral transduction of fresh mouse bone marrow cells
Murine bone marrow cells were obtained from femur and tibias. Red blood cells were lysed by treatment with ACK lysing buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM EDTA) for 2 min at room temperature. Remaining cells were washed with RPMI containing 10% fetal bovine serum, 1% penicillin/streptomycin. The cells were washed again and suspended in complete growth media (RPMI 1640 containing 20% FBS, 1% penicillin/streptomycin, 5 ng/ml mIL-3, 10 ng/ml mIL-6, 50 ng/ml mSCF, 50 ng/ml mFlt3L; Peprotech, USA), then incubated overnight. Next day, the cultured bone marrow cells were transduced with retroviral vectors by spin infection (2,500 rpm, 90 min at room temperature) in 6 well plates in the presence of 2 μg/ml polybrene (Sigma, USA).
Establishment of cell line and in vitro proliferation assay
The cells tranduced with PIM2-IRES-GFP/MYC-IRES-tNGFR, IRES-GFP/MYC-IRES-tNGFR and PIM2-IRES-GFP/IRES-tNGFR retroviral vectors were maintained initially in the presence of cytokine mixture (5 ng mIL-3, 10 ng mIL-6, 50 ng mSCF, 50 ng mFlt3L). To establish permanent cell lines, cytokines were withdrawn from the culture at day 14, and the cells were further maintained for 30 days and frozen in nitrogen tank. For proliferation assay, the numbers of live cells were determined by trypan-blue dye exclusion at different time points. At the same time, the percentage of cells expressing GFP and tNGFR (Robbins et al., 1997) were followed by FACS analysis after NGFR staining at the same time. The absolute numbers of PIM2/MYC, GFP/MYC, and PIM2/tNGFR double-positive cells were calculated by multiplying the percentage of FL1/FL2 positive population and the live cell counts. The dilution factors at different time points of culture were multiplied to derive absolute number of cells at particular time points.
Measurement of apoptotic cells
Live cells from the established myeloid cell lines that simultaneously express PIM2/MYC or MYC alone were separated by centrifugation (3,000 rpm, 20 min at room temperature) on the Ficoll-paque (GE healthcare Life Sciences, USA) layer. Live cells were purified and seeded in 12 well plates with complete culture medium, and maintained in CO2 incubator. At each time points, cells were harvested and stained with 20 μg/ml 7-amino-actinomycin-D (7-AAD; Sigma Aldrich, USA) and analyzed by FACS to enumerate (Philpott et al., 1996) apoptotic cell fractions. Triplicate wells were analyzed for each time point.
Western immunoblotting
The cells were lysed and proteins were extracted in a TNN lysis buffer (1 M Tris-HCl [pH 7.4], 10% NP-40, 5 M NaCl) and protease inhibitor (cocktail of Aprotinin, Leupeptin, PMSF, AEBSF, Na3VO4, NaF). The clear lysates were obtained after centrifugation and protein concentration was measured using Bio-Rad Protein Assay Solution (Bio-Rad Laboratories, USA). The quantified lysates were separated by 10% SDS-PAGE. After transfer to nitrocellulose membrane (Hybond-C; Amersham, UK), the membrane was stained with ponceau to confirm protein transfer. The nitrocellulose membrane was then de-stained with 5% acetic acid, and blocked for 2 h at 4°C with 5% none-fat milk and 0.1% Tween-20, PBS (pH 7.4). The blocked membranes were then incubated with the appropriate primary antibodies for 2 h at room temperature. The membrane was washed three times (5 min each) with PBS containing 0.1% Tween-20, then incubated with 1:5,000 dilution of secondary antibodies conjugated with HRP for 1 h. The immune-reactive proteins were visualized with the ECL detection reagents (PerkinElmer).
Flow cytometry analysis
Surface phenotypes of cells were analyzed by FACS Calibure and Cell Quest pro software (BD bioscience). Cultured cells were washed 2 times with FACS staining buffer (PBS containing 2% FBS, 0.1% NaN3), and stained for surface markers CD271, CD11b, Gr-1, B220, CD3 with biotin-labeled antibodies and avidin-coupled Cy-7 or phycoerythrin. Antibodies were all purchased from BD bioscience.
Transplantation and mice
C57BL6.SJL mice (Jackson laboratories, Bar Harbor, ME) were kept in SPF animal facility in Hanyang University. The cells transduced with PIM2/MYC or GFP/MYC retroviral vectors were transplanted into γ - irradiated (340 cGy) mice via lateral tail vein. For each cell line, ten mice were used as host and each mouse received 5 × 105 transformed cells. In addition, each mouse received 2 × 106 normal fresh bone marrow cells for radiation protection. Mice were monitored 3 times a week for disease indication and the mice showing disease symptoms (hind leg paralysis, bent spine, loss of movement) were sacrificed by CO2 asphyxiation. The mice were further characterized by anatomical and FACS analysis. Kaplan Meier graphs for mice survival kinetics were drawn using Grapad Prism software version 5.0.
RESULTS
Construction of retroviral vectors and bone marrow transduction
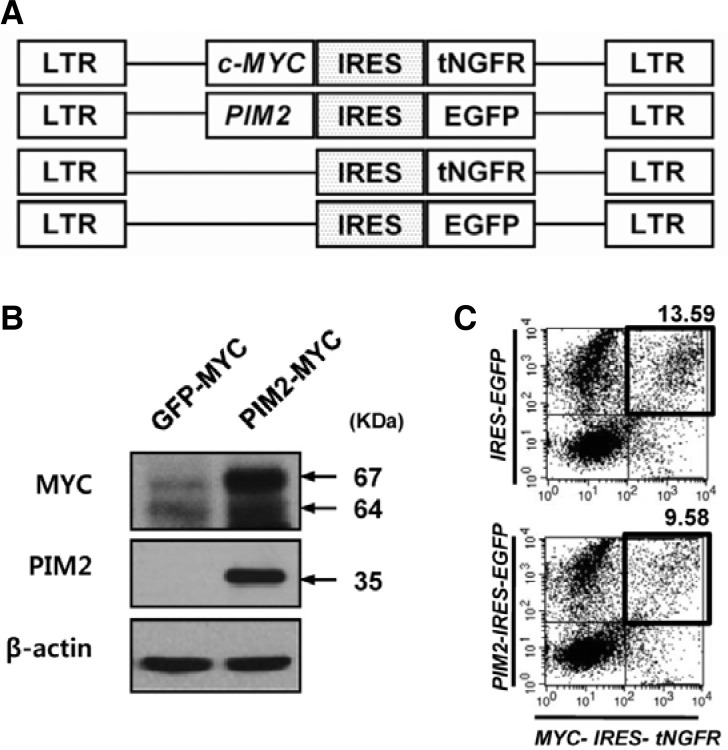
To follow cells simultaneously transduced with two retroviral vectors we constructed retroviral vectors as shown in Fig. 1A. With this combination of retroviral vectors, the MYC-transduced cells were identified by surface expression of truncated nerve growth factor receptor (tNGFR), and PIM2-transduced cells were identified by GFP expression. The constructs express expected sizes of proteins, and co-transduction of fresh mouse bone marrow cells usually results in around 10% of doubly transduced cells (Figs. 1B and 1C).
Fig. 1.
Construction of retroviral vectors and bone marrow transduction. (A) Four retroviral vectors were constructed based on the MSCV (Clontech) backbone. GFP and truncated nerve growth factor receptor genes were used as markers for MYC and PIM2 expression respectively. In both cases, internal ribosome entry sites (IRES) were used to link the proto-oncogenes with their respective markers. (B) Immunoblotting result of protein extracts obtained from MYC or PIM2/MYC-transduced cells using the antibodies against MYC or PIM2. (C) Representative results of retroviral transduction of mouse bone marrow cells. PIM2 and MYC or GFP and MYC retroviral vectors were co-infected into fresh mouse bone marrow cells. After 3 days, the cells were harvested and stained for NGFR expression (redfluorescence, FL2), and analyzed for GFP and NGFR expression.
Establishment of permanent cell lines
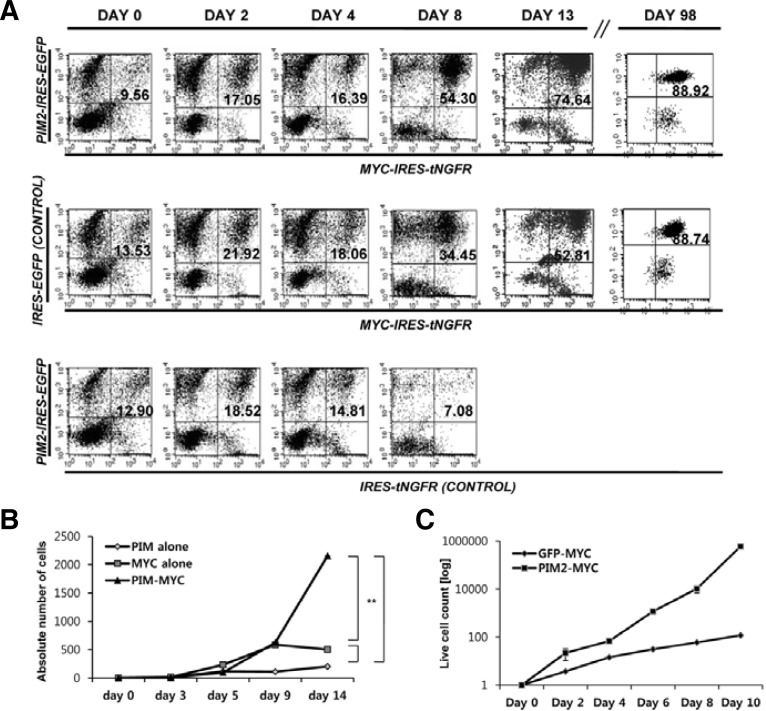
As shown in Fig. 2A, bone marrow cells transduced with MYC alone or PIM2/MYC combination, which were represented as double-positive cells in FACS results, gradually dominate the cultures as the cells are maintained in vitro in the presence of cytokine mixture (SCF, IL-6, IL-3 and Flt3-L). The bone marrow cells transduced with PIM2 alone fail to grow. The difference of growth kinetics between MYC and PIM2/MYC group become apparent from the day 9 with PIM2/MYC group showing much faster growth rate. We gradually withdrew cytokines from the day 14, and were able to maintain cells for more than three months in the absence of cytokines. At this point, the cells reach almost 90% transgene positive, and become growth factor-independent. The doubling times of PIM2/MYC and MYC-transduced cell lines were 16 and 21 h respectively (Fig. 2C).
Fig. 2.
In vitro culture of PIM2/MYC and MYC-trans-duced cells. (A) The population change observed while cells were cultured in the pre-sence of cytokine mixture. The mouse bone marrow cells transduced with PIM2/MYC, GFP/MYC, and PIM2/tNGFR were cultured in the presence of cytokine mixture (SCF, IL-3, IL-6, Flt3L). The cells expressing GFP and tNGFR simultaneously were followed by FACS analysis after NGFR staining. (B) The kinetics of cell expansion of different proto-oncogene combination groups. The numbers of live cells were measured by trypan-blue dye exclusion and the absolute number of PIM2/MYC, GFP/MYC, and PIM2/tNGFR double-positive cells were calculated by the percentage of FL1/FL2 positive population and the dilution factors at different time points of culture. (C) The growth kinetics of PIM2/MYC and MYC cell lines. The permanent cell lines established were thawed and 106 cells were used to initiate in vitro culture. The changes in absolute number of cells were plotted as described in (B).
PIM2/MYC proto-oncogene combination suppresses MYC-induced apoptosis
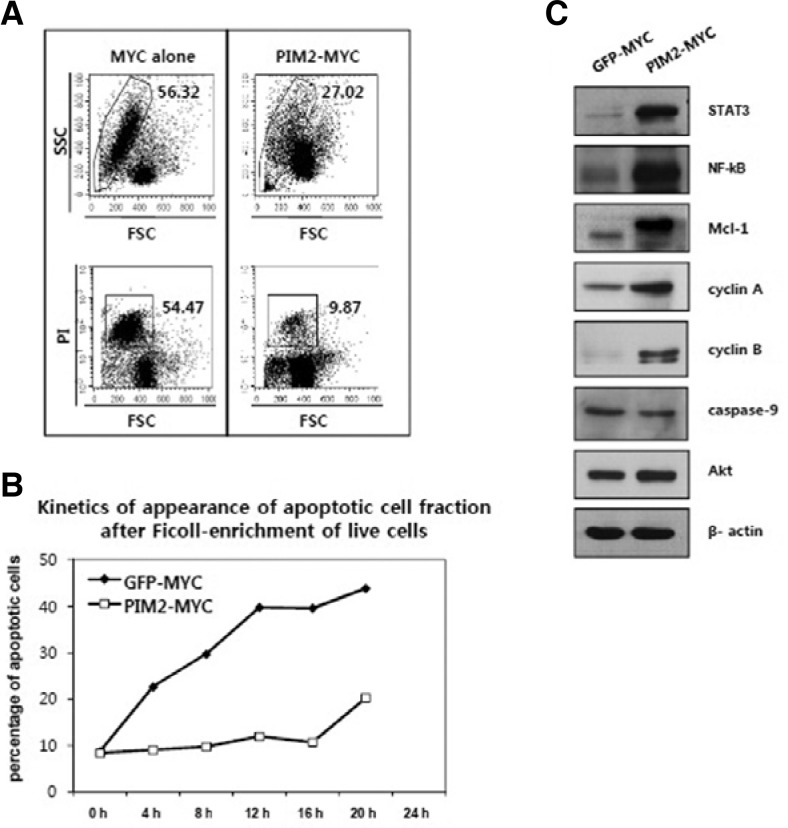
Cells transduced with PIM2/MYC consistently show fewer apoptotic cells in culture compared to the MYC alone group. MYC expression in hematopoietic cells has been shown to promote both cell division and apoptosis at the same time in previous reports. The cell line established by PIM2/MYC consistently shows fewer apoptotic cells than the MYC-transduced cell line (Fig. 3A). In Fig. 3B, live cells were first enriched by Ficoll separation, and the fractions of apoptotic cells appearing at different time points were measured by 7-AAD staining and FACS analysis. As shown in the figure, more apoptotic cells appear at earlier time points in the bone marrow cell line transformed by MYC alone than the one transformed by PIM2/MYC. Moreover, immunoblotting results show that the PIM2/MYC cell line expresses increased levels of proteins related to cell proliferation and survival such as cyclin A, cyclin B, STAT3, Mcl-1, and NF-κB (p65) compared to the cell line transduced with MYC alone. Overall, these results show that simultaneous expression of PIM2 and MYC clearly increases rates of cell expansion while inhibiting apoptosis.
Fig. 3.
PIM2 suppresses MYC-induced apoptosis in bone marrow cells. (A) The cell line established by PIM2/MYC transduction shows less apoptotic cells than MYC-transduced cell line during routine culture. The figure shows a representative profile of apoptotic cell fraction shown as forward/side scatter (FSC/SSC) or PI staining intensity versus FSC diagrams. (B) Kinetics of appearance of apoptotic cells after enrichment of live cells by Ficoll-gradient separation. Live cells were enriched by Ficoll-gradient separation, and the appearance of apoptotic cells measured by 7-AAD staining every 4 h over a one-day interval. (C) Immunoblot analysis of protein extracts obtained from the cell lines indicated.
The cells transduced with PIM2/MYC proto-oncogene combination show more immature myeloid lineage phenotype
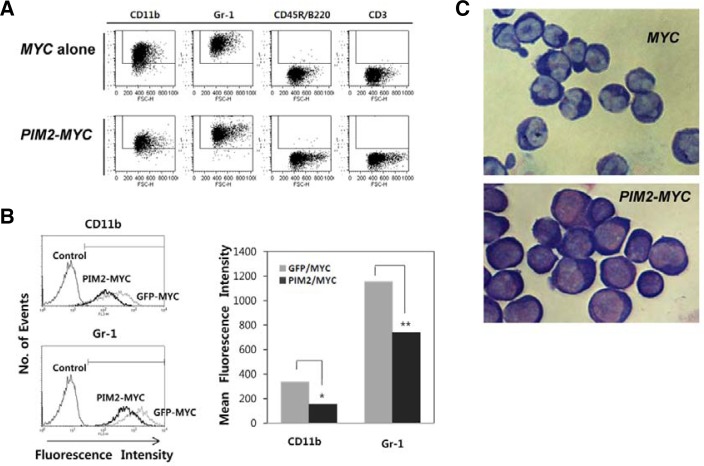
The cytokine combination we used initially in culture allows only myeloid cell expansion. As a result, both MYC and PIM2/MYC-transduced cells show myeloid lineage phenotype (CD11b+, Gr-1+, B220−, CD3−) as shown in Fig. 4A. However, PIM2/MYC-transduced cells express lower level of CD11b and Gr-1 compared to the cells transduced with MYC alone as shown in Fig. 4B. Both CD11b and Gr-1 surface makers are expressed on myeloid cells starting from a common myeloid lineage progenitor stage during hematopoietic cell differentiation in mouse bone marrow. Furthermore, the morphology of the two cell lines show subtle differences with PIM2/MYC-transduced cells showing monoblast/promyelocyte-like morphology and the MYC-transduced cells showing promyelocyte-metamyelocyte morphology (Fig. 4C). These results indicate that PIM2, when co-expressed with MYC, blocks myeloid cell differentiation at some point between the monoblast and promyelocyte stages of myeloid cell differentiation in mouse bone marrow.
Fig. 4.
The cell line established by PIM2/MYC transduction expresses myeloid lineage markers with a more undifferentiated phenotype than the MYC-transduced cell line. (A) The cell lines established show myeloid phenotype. The cells are stained with the antibodies against the differentiation markers indicated and analyzed on flow cytometer. (B) Comparative intensities of CD11b and Gr-1 surface markers. (C) Giemsa-Wright stain of the two cell lines. Cells were centrifuged onto glass slides by cytospin, and stained with Giemsa-Wright stain.
The PIM2/MYC-transduced, but not MYC-transduced cells, induce myeloid tumors upon in vivo transplantation
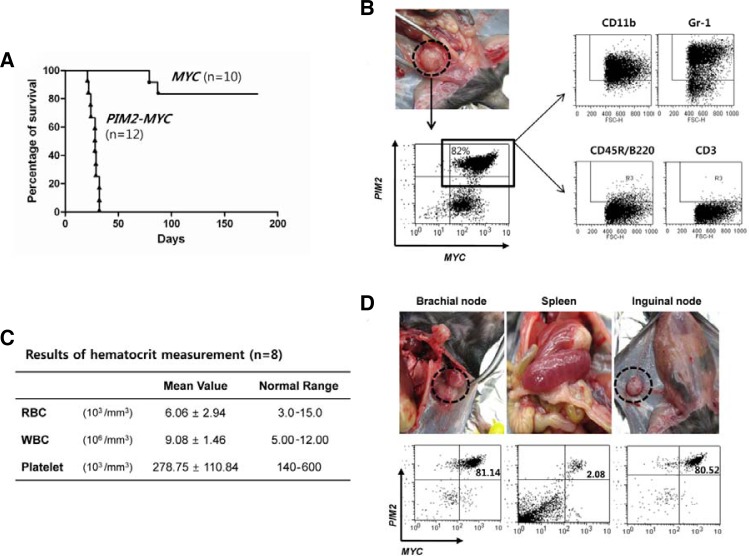
Next we tested whether the cells transformed and established in vitro still maintain their in vivo tumorigenic potential. The cell lines established by PIM2/MYC and MYC were injected into syngeneic host mice conditioned with a minimal dose of γ-irradiation (340 cGy). As shown in Fig. 5A, all the mice injected with the PIM2/MYC-transformed cell line succumbed to malignant myeloid sarcoma between 25 to 35 days after injection. On the other hand, 80% of mice that received the MYC-transformed cell line remained healthy even after 90 days. Figure 5B shows one representative mouse harboring large myeloid sarcomas nodules beneath the spine. The cells obtained from the tumor mass exhibit the same surface phenotype as the cells initially injected as shown in the FACS profile. Figure 5C shows the summary of blood hematocrit data collected from the eight mice showing apparent signs of hematopoietic solid tumors such as reduced agility, hunched back, weight-loss and hind-leg paralysis. In all the mice with solid tumors, however, examination of the cellular composition of blood revealed a normal distribution of phenotypically mature cells, with no evidence of circulating blastic cells indicating that the hematopoietic cancers are not leukemic, but of solid type such as sarcoma or lymphoma. This is consistent with the autopsy data presented in Fig. 5D, wherein it is shown that the nodular sarcomas are mostly composed of PIM2/MYC-transduced cells, and furthermore very few PIM2/MYC-transduced cells were detected in the spleen indicating tumor cells in these mice are not circulating as we would expect to observe in leukemia. Instead, the tumor cells are mostly limited to lymph nodes where they form solid myeloid sarcomas.
Fig. 5.
PIM2/MYC-transduced cells form myeloid sarcomas upon in vivo injection. (A) The mice received 5 × 105 cells of the cell lines establised with the gene(s) indicated. Ten (MYC) and 12 mice (PIM2/MYC) were used as hosts as described in “Materials and Methods”. Eight mice from the MYC-alone group were healthy and survived for more than 150 days at which time all the mice were sacrificed. (B) A representative sarcoma that developed beneath the spine. Phenotypic analysis of sarcoma cells demonstrated the majority to still be positive for GFP and tNGFR markers and clearly of myeloid phenotype similar to the transplanted parental cells. (C) The hematocrit data for symptomatic mice are demonstrated to be within normal limits. The table shows the average hematocrit collected from eight mice that have received cells from PIM2/MYC cell line. (D) Sarcomas are formed in lymph nodes and are mostly composed of cells still expressing the markers for MYC (tNGFR) and PIM2 (GFP) expression similarly to the parental cells that were transplanted to the mice while minimal number of double-positive cells are detected in spleen. The same is true for bone marrow (data not presented).
DISCUSSION
In this report, we show that PIM2/MYC co-transduction of fresh mouse bone marrow cells readily establishes permanent myeloid cell lines that are capable of in vivo tumor formation. The bone marrow cell line established by transformation with MYC alone does not form tumors in vivo although it is sufficient to establish an in vitro myeloid cell line. The cooperation between PIM2 and MYC in cellular transformation has been documented before in a bi-transgenic mouse model (Allen et al., 1997) and in other somatic tumor models such as prostate carcinoma (Chen et al., 2005). In fact, PIM increases MYC transcriptional activity by phosphorylation of S62 (by PIM-1) and S329 (by PIM-2) of MYC resulting in stabilization of MYC (Zhang et al., 2008) so that the transcriptional activity of MYC can be maintained constitutively. In addition, Pim kinases have been shown to induce relocation of p21, p27, and phosphorylation of BAD resulting in increased cell proliferation and reduced apoptosis (Wang et al., 2010; Yang et al., 2011).
Although various molecular events during PIM/MYC collaboration have been studied, the early events of malignant transformation of normal cells by PIM/MYC has been difficult to study since the transformation events occurring in vivo are very rare. Since PIM2 expression is frequently up-regulated in myeloid leukemic cells in patients (Mizuki et al., 2003), and since our preliminary experiments show that expression of the PIM2/MYC combination of proto-oncogenes will induce AML as well as B lineage lymphomas in a mouse model of gene transduction and transplantation of bone marrow cells, we decided to establish a malignant transformation model in vitro using fresh bone marrow myeloid cells. In this report, we showed that PIM2/MYC co-transduction of bone marrow cells establishes malignantly transformed myeloid cell lines in culture. The transformed cells in this system become growth factor-independent upon prolonged culture and form lethal myeloid sarcomas upon injection into syngeneic host mice. In a previous report on a bi-transgenic model of PIM2/MYC, in which MYC transgene expression was driven by Eμ-enhancer, only B and T cell lymphomas were observed (Allen et al., 1997). In our own transduction-transplantation experiment, in which PIM2/MYC-transduced bone marrow cells were immediately injected into host mice after transduction, without further in vitro culture, we also observed only B-lineage leukemia/lymphoma in host mice. However, we consistently observed transgene-positive myeloid cells at early stage of leukemic host mice, and these presumably leukemic myeloid cells disappeared upon serial transfer of host bone marrow cells into successive generations of recipient mice indicating that B-lineage leukemic and lymphoma cells might be more dominant in this environment. These previous results by others combined with observations in our bone marrow transduction/transplantation model prompted us to test whether PIM2/MYC proto-oncogene combination can transform fresh myeloid precursors into genuinely malignant cancer cells in vitro.
In this report, we clearly showed that PIM2/MYC proto-oncogene combination can readily transform mouse bone marrow myeloid cells, and the transformed cells become factor-independent at very early stages of culture (2–3 wks). Moreover, these cells establish lethal myeloid sarcoma upon in vivo administration indicating that these cells are malignantly transformed. One intriguing finding in this study is that the cells transformed with MYC alone do not form in vivo leukemias or solid tumors even though the MYC-transformed cells become factor-independent and will establish permanent cell lines. This result contrasts with previous report in which MYC-transduced bone marrow cells efficiently induce AML upon transfer into recipient mice (Luo et al., 2005). Our own preliminary experiments also show that mouse bone marrow cells transduced with MYC efficiently induced AML as well as B cell leukemia and lymphoma upon transfer into syngeneic host mice. We believe that the MYC-transduced cells lose the malignant phenotype while they are maintained in an in vitro culture system for prolonged period, and therefore, comparisons between MYC-transformed cells at different time points during successive generations in culture may reveal critical parameters that determine malignant phenotype.
We also report that our PIM2/MYC-transduced cell line exclusively induces sarcoid myeloid cancers which are demonstrably devoid of circulating leukemic cells. As shown in Fig. 5, these mice succumb to myeloid sarcomas rapidly starting from day 20 after injection. Blood hematocrit data of representative mice all show normal hematocrit at the time of sacrifice, and a minimal number (between 2 to 4%) of circulating leukemic cells are observed in spleen and bone marrow. Previous results from a transduction/transplantation study conducted in our lab using cells similarly transduced with the same gene combination, and then immediately re-introduced into mice after viral infection, showed leukemic cells as well as sarcoma/lymphoma type of cells in the recipient mice. Therefore, we think the system introduced in this study provides a novel opportunity to examine parameters that determine circulating leukemic versus localized sarcoma presentations in hematopoietic malignancies.
We believe that this report is the first to show directly that the PIM2/MYC combination of proto-oncogenes exerts a potent synergy sufficient to drive myeloid cell proliferation and immortalization resulting in a permanent cell line which can survive independent of growth factors. Furthermore we have demonstrated that on transplantation to syngeneic mice, the cell line will only form lethal sarcomas in vivo, with nearly undetectable numbers of circulating leukemic cells evident. In humans, myeloid sarcomas, also called chloromas, are a very rare presentation in leukemic malignancies or pre-leukemic states, and are most commonly associated with myeloproliferative or myelodysplastic syndromes in progression to genuine leukemia (Deme et al., 1997). Since our model exclusively forms myeloid sarcomas, it is likely to prove useful in studying molecular processes of transformation leading to myeloid sarcoma development.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number: 2011-0027204). This research was supported by National Nuclear R&D Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant number: 2012-0004890).
REFERENCES
- Allen J.D., Verhoeven E., Domen J., van der Valk M., Berns A. Pim-2 transgene induces lymphoid tumors, exhibiting potent synergy with c-myc. Oncogene. 1997;15:1133–1141. doi: 10.1038/sj.onc.1201288. [DOI] [PubMed] [Google Scholar]
- Brown D., Kogan S., Lagasse E., Weissman I., Alcalay M., Pelicci P.G., Atwater S., Bishop J.M. A PMLRA-Ralpha transgene initiates murine acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman J.D., Lapidot T., Wang J.C., Doedens M., Shultz L.D., Lansdorp P., Dick J.E., Eaves C.J. Kinetic evidence of the regeneration of multilineage hematopoiesis from primitive cells in normal human bone marrow transplanted into immunodeficient mice. Blood. 1997;89:4307–4316. [PubMed] [Google Scholar]
- Chen W.W., Chan D.C., Donald C., Lilly M.B., Kraft A.S. Pim family kinases enhance tumor growth of prostate cancer cells. Mol. Cancer Res. 2005;3:443–451. doi: 10.1158/1541-7786.MCR-05-0007. [DOI] [PubMed] [Google Scholar]
- Cheng G.X., Zhu X.H., Men X.Q., Wang L., Huang Q.H., Jin X.L., Xiong S.M., Zhu J., Guo W.M., Chen J.Q., et al. Distinct leukemia phenotypes in transgenic mice and different corepressor interactions generated by promyelocytic leukemia variant fusion genes PLZF-RARalpha and NPM-RARalpha. Proc. Natl. Acad. Sci. USA. 1999;96:6318–6323. doi: 10.1073/pnas.96.11.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenco G.M., Nucifora G., Ren R. Human AML1/MDS1/EVI1 fusion protein induces an acute myelogenous leukemia (AML) in mice: a model for human AML. Proc. Natl. Acad. Sci. USA. 2000;97:1760–1765. doi: 10.1073/pnas.030421197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deme S., Deodhare S.S., Tucker W.S., Bilbao J.M. Granulocytic sarcoma of the spine in nonleukemic patients: report of three cases. Neurosurgery. 1997;40:1283–1287. doi: 10.1097/00006123-199706000-00031. [DOI] [PubMed] [Google Scholar]
- Dick J.E., Bhatia M., Gan O., Kapp U., Wang J.C. Assay of human stem cells by repopulation of NOD/SCID mice. Stem Cells. 1997;15(Suppl 1):199–203. doi: 10.1002/stem.5530150826. discussion 204–197. [DOI] [PubMed] [Google Scholar]
- Grisolano J.L., Sclar G.M., Ley T.J. Early myeloid cell-specific expression of the human cathepsin G gene in transgenic mice. Proc. Natl. Acad. Sci. USA. 1994;91:8989–8993. doi: 10.1073/pnas.91.19.8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisolano J.L., Wesselschmidt R.L., Pelicci P.G., Ley T.J. Altered myeloid development and acute leukemia in transgenic mice expressing PML-RAR alpha under control of cathepsin G regulatory sequences. Blood. 1997;89:376–387. [PubMed] [Google Scholar]
- Grisolano J.L., O’Neal J., Cain J., Tomasson M.H. An activated receptor tyrosine kinase, TEL/PDGFbetaR, cooperates with AML1/ETO to induce acute myeloid leukemia in mice. Proc. Natl. Acad. Sci. USA. 2003;100:9506–9511. doi: 10.1073/pnas.1531730100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M.L., Swiderski C.F., Howard D.S., Grimes B.A., Rossi R.M., Szilvassy S.J., Jordan C.T. Preferential induction of apoptosis for primary human leukemic stem cells. Proc. Natl. Acad. Sci. USA. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen P.O., Sorensen D.R., Benestad H.B. Inhibitors of angiogenesis selectively reduce the malignant cell load in rodent models of human myeloid leukemias. Leukemia. 2002;16:376–381. doi: 10.1038/sj.leu.2402376. [DOI] [PubMed] [Google Scholar]
- Kroon E., Krosl J., Thorsteinsdottir U., Baban S., Buchberg A.M., Sauvageau G. Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. EMBO J. 1998;17:3714–3725. doi: 10.1093/emboj/17.13.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T., Fajerman Y., Kollet O. Immune-deficient SCID and NOD/SCID mice models as functional assays for studying normal and malignant human hematopoiesis. J. Mol. Med. 1997;75:664–673. doi: 10.1007/s001090050150. [DOI] [PubMed] [Google Scholar]
- Lavau C., Luo R.T., Du C., Thirman M.J. Retrovirus-mediated gene transfer of MLL-ELL transforms primary myeloid progenitors and causes acute myeloid leukemias in mice. Proc. Natl. Acad. Sci. USA. 2000;97:10984–10989. doi: 10.1073/pnas.190167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence H.J., Rozenfeld S., Cruz C., Matsukuma K., Kwong A., Komuves L., Buchberg A.M., Largman C. Frequent co-expression of the HOXA9 and MEIS1 homeobox genes in human myeloid leukemias. Leukemia. 1999;13:1993–1999. doi: 10.1038/sj.leu.2401578. [DOI] [PubMed] [Google Scholar]
- Luo H., Li Q., O’Neal J., Kreisel F., Le Beau M.M., Tomasson M.H. c-Myc rapidly induces acute myeloid leukemia in mice without evidence of lymphoma-associated antiapoptotic mutations. Blood. 2005;106:2452–2461. doi: 10.1182/blood-2005-02-0734. [DOI] [PubMed] [Google Scholar]
- Mizuki M., Schwable J., Steur C., Choudhary C., Agrawal S., Sargin B., Steffen B., Matsumura I., Kanakura Y., Bohmer F.D., et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood. 2003;101:3164–3173. doi: 10.1182/blood-2002-06-1677. [DOI] [PubMed] [Google Scholar]
- Ory D.S., Neugeboren B.A., Mulligan R.C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott N.J., Turner A.J., Scopes J., Westby M., Marsh J.C., Gordon-Smith E.C., Dalgleish A.G., Gibson F.M. The use of 7-amino actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared with other techniques. Blood. 1996;87:2244–2251. [PubMed] [Google Scholar]
- Robbins P.B., Yu X.J., Skelton D.M., Pepper K.A., Wasserman R.M., Zhu L., Kohn D.B. Increased probability of expression from modified retroviral vectors in embryonal stem cells and embryonal carcinoma cells. J. Virol. 1997;71:9466–9474. doi: 10.1128/jvi.71.12.9466-9474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid I., Uittenbogaart C.H., Keld B., Giorgi J.V. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J. Immunol. Methods. 1994;170:145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- Thorsteinsdottir U., Krosl J., Kroon E., Haman A., Hoang T., Sauvageau G. The oncoprotein E2A-Pbx1a collaborates with Hoxa9 to acutely transform primary bone marrow cells. Mol. Cell. Biol. 1999;19:6355–6366. doi: 10.1128/mcb.19.9.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Kimura T., Asada R., Harada S., Yokota S., Kawamoto Y., Fujimura Y., Tsuji T., Ikehara S., Sonoda Y. SCID-repopulating cell activity of human cord blood-derived CD34- cells assured by intra-bone marrow injection. Blood. 2003;101:2924–2931. doi: 10.1182/blood-2002-09-2782. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhang Y., Gu J.J., Davitt C., Reeves R., Magnuson N.S. Pim-2 phosphorylation of p21(Cip1/WAF1) enhances its stability and inhibits cell proliferation in HCT116 cells. Int. J. Biochem. Cell Biol. 2010;42:1030–1038. doi: 10.1016/j.biocel.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga P.K., Setroikromo R., Kamps G., Kampinga H.H., Vellenga E. Differences in heat sensitivity between normal and acute myeloid leukemic stem cells: feasibility of hyperthermic purging of leukemic cells from autologous stem cell grafts. Exp. Hematol. 2003;31:421–427. doi: 10.1016/s0301-472x(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Yang H., Wang Y., Qian H., Zhang P., Huang C. Pim protein kinase-3 is regulated by TNF-alpha and promotes endothelial cell sprouting. Mol. Cells. 2011;32:235–241. doi: 10.1007/s10059-011-1026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O.H., Valdez R., Theisen B.K., Guo W., Ferguson D.O., Wu H., Morrison S.J. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhou L., Miyamoto T., Iwasaki H., Harakawa N., Hetherington C.J., Burel S.A., Lagasse E., Weissman I.L., Akashi K., et al. AML1-ETO expression is directly involved in the development of acute myeloid leukemia in the presence of additional mutations. Proc. Natl. Acad. Sci. USA. 2001;98:10398–10403. doi: 10.1073/pnas.171321298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Grindley J.C., Yin T., Jayasinghe S., He X.C., Ross J.T., Haug J.S., Rupp D., Porter-Westpfahl K.S., Wiedemann L.M., et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Z., Li X., Magnuson N.S. Pim kinase-dependent inhibition of c-Myc degradation. Oncogene. 2008;27:4809–4819. doi: 10.1038/onc.2008.123. [DOI] [PubMed] [Google Scholar]