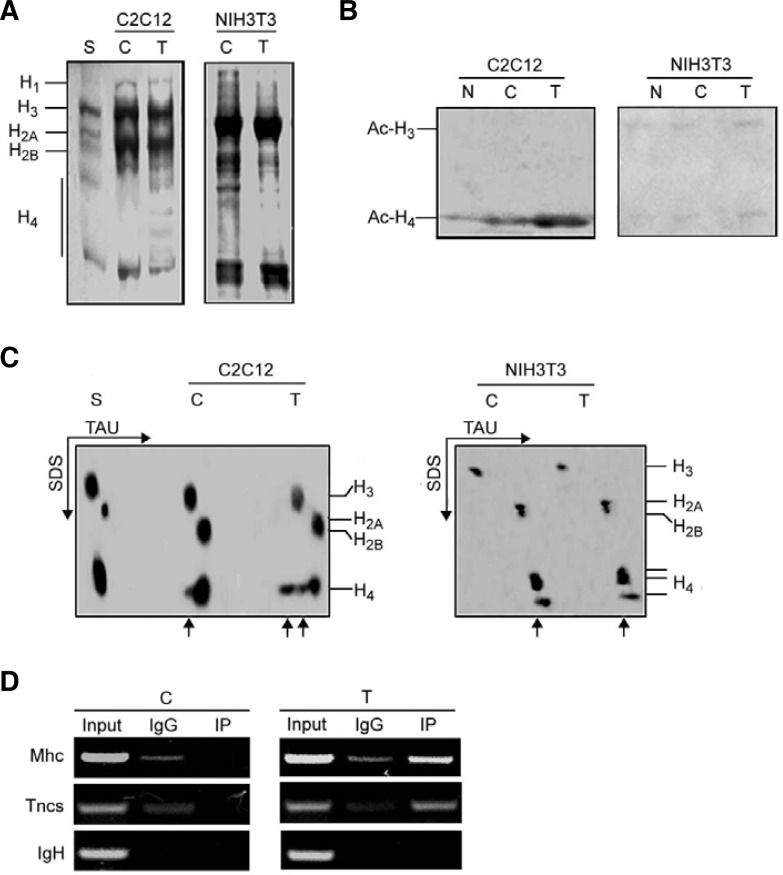
Fig. 7.
Overexpression of Myog increases hyperacetylation of histone H4 in differentiating C2C12 myoblasts but not NIH3T3 fibroblasts. NIH3T3 fibroblasts and C2C12 myoblasts were transfected with pEMSV-Myog or empty vector. After 24 h of transfection, cells were induced to differenttiate for 48 h. After harvest the nuclei were isolated, and histones were extracted as described in the “Materials and Methods.” (A) Analysis of histones by 15% SDS-PAGE. The gel was stained with Coomassie brilli-ant blue. (B) Western blot analysis of acetylated histone H3 and H4. Histones (60 μg) were separated by 15% SDS-PAGE and probed with antibodies against acetylated N-terminal sequences of H3 and H4. (C) Hyperacetylation of histones in 2-D gel electrophoresis. Histones (60 μg) were separated on TAU/SDS (2-D) gel using triton-acetic acid-urea (TAU) minislab gels for the first direction and SDS minislab gels for the second direction. The gel was stained with Coomassie brilliant blue. The arrows indicate mono- and multi-acetylated histones. (D) Recruitment of acetylated-histone H4 to the promoter regions of late muscle genes in control or Myogtransfected C2C12 myoblasts was analyzed by ChIP assay. After transfection, the chromatins were immuno-precipitated using anti-acetylated–histone H4 antibody or control IgG. The specific promoter sequences of late muscle genes were amplified by PCR. The IgH enhancer containing E boxes was used as negative control. S, standard histones (calf thymus histones); C, control (empty vector transfection); T, transfection; N, no transfection.