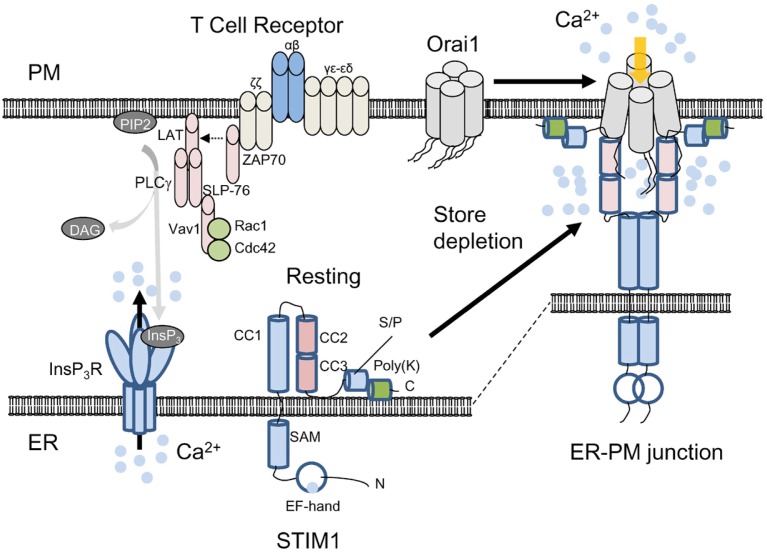
Fig. 2.
Activation mechanism of Orai1 and STIM1. Schematic showing current understanding of CRAC channel activation. Under resting conditions, Orai1 and STIM1 are distributed at the PM and the ER membrane. The subunit stoichiometry of Orai1 under resting and stimulated conditions is currently unclear. For convenience, a tetrameric assembly of Orai1 is depicted here. Upon store depletion triggered by T cell receptor stimulation and InsP3 production via PLCγ1, STIM1 oligomerizes by sensing ER Ca2+ depletion with its ER-luminal EF-hand domain, clusters and translocates to the ER-PM junctions. By physical interaction with the cytoplasmic, N and C terminus of Orai1 through the CAD/SOAR domain (coiled coil domains 2 and 3), clustered STIM1 recruits and activates Orai1 in the ER-PM junctions. STIM1 contains an ER-luminal region comprising the EF-hand and SAM domains, a single transmembrane segment, and a cytoplasmic region. The cytoplasmic region has three coiled-coil domains (CC1, 2, and 3), serine/proline-rich domain (S/P) containing the residues involved in posttranslational modifications (see below), and a polybasic region (poly-K) at the C terminus that interacts with phosphoinositides after store depletion. The exact role of the poly-K tail of STIM1 in resting conditions is not known, but it may interact with phosphoinositides on the ER membrane to maintain its inactive, folded structure.