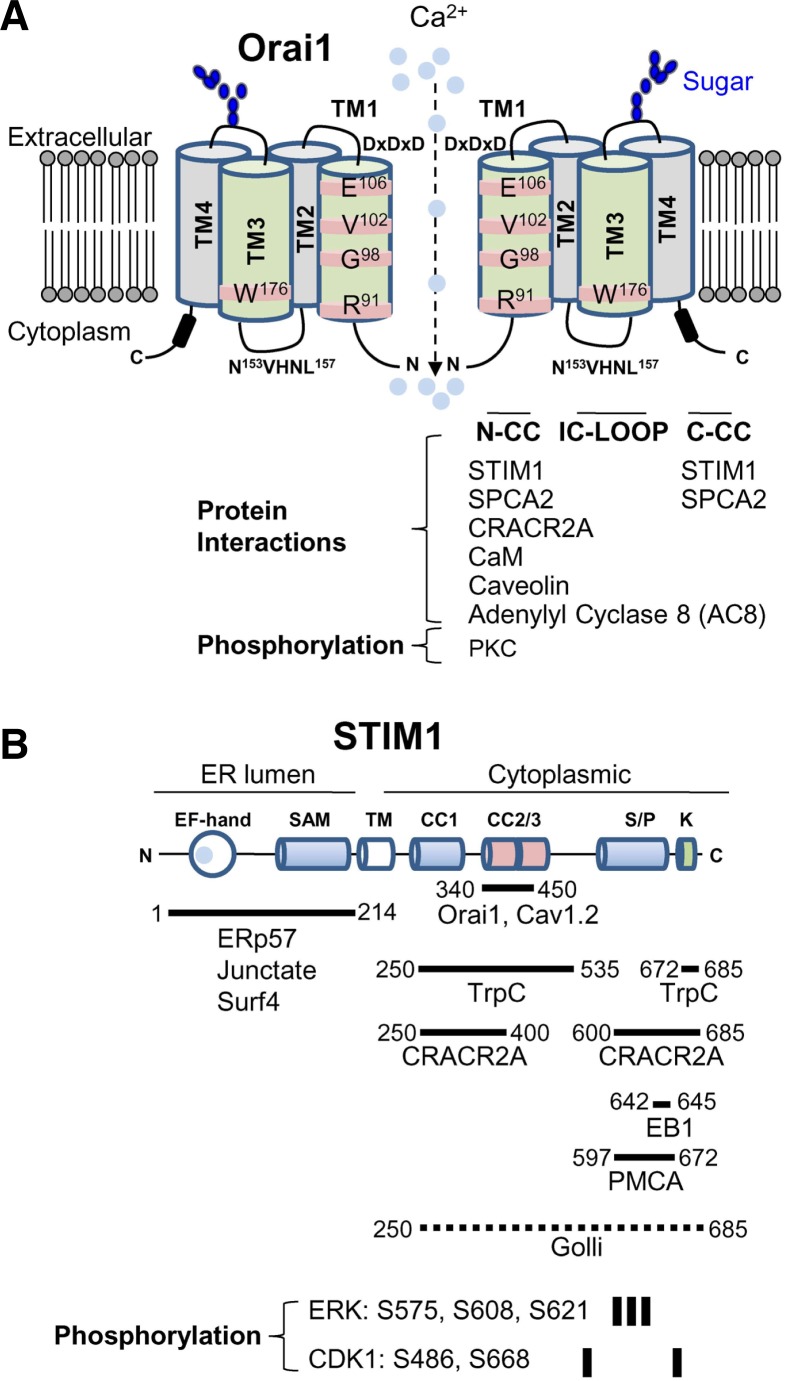
Fig. 3.
Interacting partners and posttranslational modification of Orai1 and STIM1. (A) Schematic of Orai1. Orai1 has four transmembrane segments (TM1-TM4). It has two extracellular domains and the second extracellular domain between TM3 and TM4 contains the asparagine (N223) residue involved in glycosylation (indicated in blue). The TM1 lines the pore and the residues in TM1 involved in Ca2+ selectivity and gating are depicted. The TM3 does not line the pore, but affects ion selectivity by possible interaction with TM1. Subunits of Orai1 form CRAC channels, but the molecular stoichiometry of CRAC channels is still in question. Schematic depicts a dimeric form for convenience of drawing. Orai1 contains three intracellular domains including the N terminus (N-CC), intracellular loop (IC-LOOP), and C-terminal coiled-coil domain (C-CC) that are important for protein interactions and channel activation/inactivation. Known molecular interactors of Orai1 in these intracellular domains are summarized. The N terminus of Orai1 is phosphorylated by protein kinase C (PKC). (B) Schematic of STIM1. STIM1 contains Ca2+-binding EF hands and a sterile α motif (SAM) domain in the ER-luminal region, a single transmembrane segment, and a long cytoplasmic region. The cytoplasmic region has three coiled-coil domains, serine/proline-rich domain, and a polybasic segment at the C terminus. Proteins associating with each of these domains are indicated. The fragment of STIM1 (340-450) involved in Orai1 interaction/gating is indicated. Golli proteins interact with the cytoplasmic region of STIM1, but the detailed interaction domain is not determined (dotted line). Residues phosphorylated by extracellular signal-regulated kinase (ERK) and cyclin-dependent kinase (CDK1) are indicated.