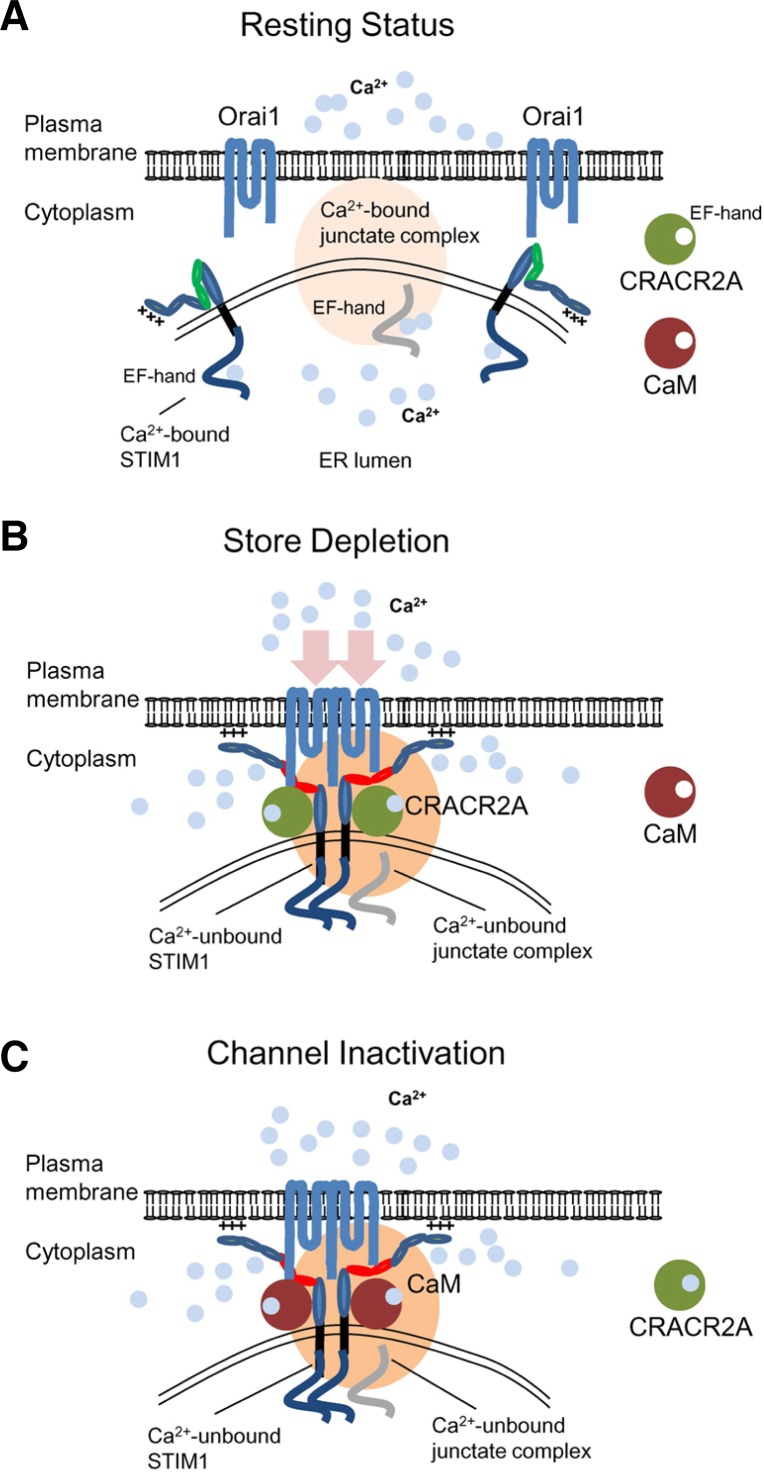
Fig. 4.
CRAC channel regulation by multiple Ca2+-sensing molecules in the ER and cytoplasm. (A) Schematic showing a possible mechanism of CRAC channel regulation. Under resting conditions, Orai1 and STIM1 are distributed at the PM and the ER membrane. Junctate is located at ER-PM junctions in a Ca2+-bound form via its ER-luminal EF hand domain (indicated in gray). Cytoplasmic Ca2+ sensors such as CRACR2A and calmodulin are not bound to Ca2+ in resting conditions. (B) Upon store depletion, STIM1 oligomerizes by sensing ER Ca2+ depletion with its ER-luminal EF-hand domain, and translocates to form clusters at the ER-PM junctions. By physical interactions with Orai1 through the CAD/SOAR domain (depicted in red), clustered STIM1 recruits and activates Orai1 in the ER-PM junctions. During the process, junctate loses bound Ca2+ and supports STIM1 recruitment into ER-PM junctions. CRACR2A is recruited into the Orai1-STIM1 complex to stabilize their interactions. (C) Following the increase of cytoplasmic [Ca2+], CRACR2A dissociates from the Orai1-STIM1 complex. Ca2+-bound calmodulin interacts with the N terminus of Orai1 and inactivates the channel via a mechanism called fast inactivation. The slow inactivation of CRAC channels depends on Ca2+ entry and interaction with SARAF. After channel inactivation, once the ER is refilled with Ca2+, Orai1 and STIM1 return to the resting status.