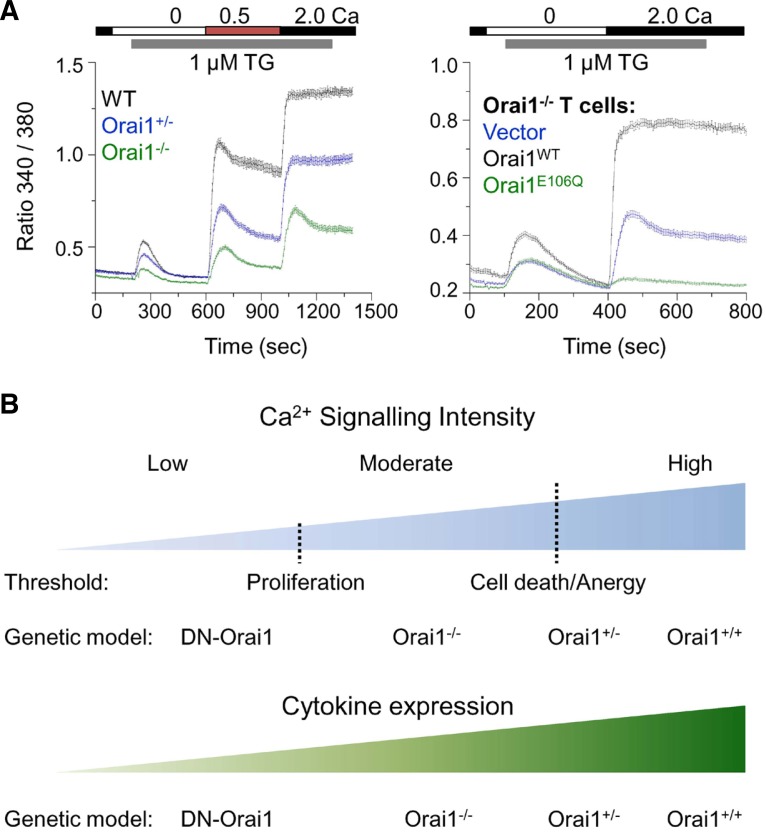
Fig. 5.
Roles of Ca2+ signalling in diverse aspects of T cell activation. (A) Gradual levels of store-operated Ca2+ entry generated by genetic modifications. Left-CRAC channel activity was measured in effector CD4+ T cells from wild type (WT), Orai1 heterozygous (Orai1+/−) and Orai1-deficient (Orai1−/−) mice after store depletion with thapsigargin (TG) in the presence of extracellular solution containing 0.5 and 2 mM Ca2+. Right-CRAC channel activity was measured in Orai1−/− CD4+ T cells transduced with retroviruses expressing empty vector (vector, blue trace), wild type (Orai1WT, black trace) or dominant negative mutant of Orai1 (Orai1E106Q). Data modified from article originally published in (Kim et al., 2011). (B) Ca2+ requirement for T cell death, cytokine production and proliferation differs. T cell proliferation does not need high Ca2+. Instead, excessive Ca2+ concentrations induce cell death and anergy in T cells. Therefore, T cell proliferation requires moderate intracellular Ca2+ concentrations as observed in Orai1-deficient (Orai1−/−) T cells and a further reduction in [Ca2+]i by overexpression of dominant negative Orai1 (DN-Orai1) in Orai1−/− T cells inhibits proliferation. Thus the threshold of [Ca2+]i necessary for proliferation is much lower than that for cell death and anergy. However, cytokine production gradually increases with increase in [Ca2+]i, with DN-Orai1 cells showing minimal cytokine production and Orai1+/+ cells showing maximal cytokine levels. Thus, the pattern of Ca2+ signalling can modulate the outcomes of T cell fates such as cytokine production, proliferation, anergy, and cell death in a digital or analogue manner.