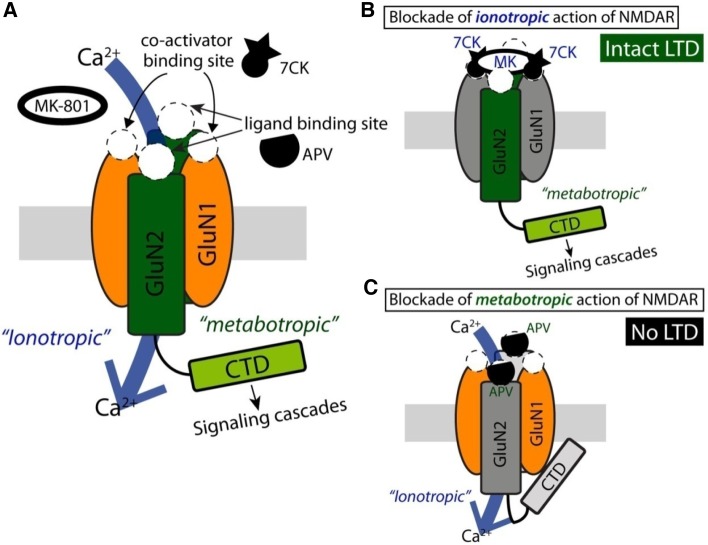
Fig. 1.
Schematic diagram of a new model that depicts ionotropic and metabotropic actions of NMDARs. (A) NMDARs are tetramers, composed of 2 GluN1 subunits and 2 GluN2 subunits. The GluN1 subunit contains glycine-binding sites that can be antagonized by 7CK. The GluN2 subunit contains glutamate-binding sites that can be antagonized by APV and R-CPP. MK-801 is a pore blocker that eliminates ion flux through NMDARs in a use-dependent manner as ketamine. The new model proposes that upon glutamate binding and release of the Mg2+ block, NMDARs allow ion flux and initiate signaling cascades, presumably via the C-terminal domain (CTD). (B) When ionotropic action is selectively inhibited by antagonists such as 7CK, MK-801, and ketamine, NMDAR-dependent LTD in the hippocampus is expected to be intact. (C) When the metabotropic action of NMDAR is selectively inhibited using competitive antagonists such as APV and R-CPP, NMDAR-dependent LTD is anticipated to be eliminated due to blockade of the necessary signaling cascades.