Abstract
Neural epidermal growth factor-like protein-like 2 (NELL2) is a secreted glycoprotein that is predominantly expressed in the nervous system, but little is known about the intracellular movement and secretion mechanism of this protein. By monitoring the localization and movements of enhanced green fluorescent protein (EGFP)-labeled NELL2 in living cultured hippocampal neuroprogenitor HiB5 cells, we determined the subcellular localization of NELL2 and its intracellular movement and secretion mechanism. C-terminal EGFP-fused NELL2 showed a typical expression pattern of secreted proteins, especially with respect to its localization in the endoplasmic reticulum, Golgi apparatus, and punctate structures. Vesicles containing NELL2 exhibited bidirectional movement in HiB5 cells. The majority of the vesicles (70.1%) moved in an anterograde direction with an average velocity of 0.454 μm/s, whereas some vesicles (28.7%) showed retrograde movement with an average velocity of 0.302 μm/s. The movement patterns of NELL2 vesicles were dependent upon the presence of microtubules in HiB5 cells. Anterograde movement of NELL2 did not lead to a detectable accumulation of NELL2 in the peripheral region of the cell, indicating that it was secreted into the culture medium. We also showed that the N-terminal 29 amino acids of NELL2 were important for secretion of this protein. Taken together, these results strongly suggest that the N-terminal region of NELL2 determines both the pattern of its intracellular expression and transport of NELL2 vesicles by high-velocity movement. Therefore, NELL2 may affect the cellular activity of cells in a paracrine or autocrine manner.
Keywords: glycoprotein, intracellular movement, NELL2, secretion
INTRODUCTION
nel was identified as a chicken neural epidermal growth factor-like protein from a chick embryo-derived cDNA library. Interestingly, although it is expressed in all tissues during fetal development, expression of its mRNA is retained only in neural tissues after hatching (Matsuhashi et al., 1995). Two human and rat cDNAs were later isolated as NELL1 and NELL2 (nel-like genes), with 50% and 80% homology to nel (Kuroda et al., 1999; Luce and Burrows, 1999; Watanabe et al., 1996). Neural tissue-specific expression of NELL2 has been reported in developing and post-natal rats (Kim et al., 2002; Kuroda et al., 1999; Nelson et al., 2004; Oyasu et al., 2000) and humans (Watanabe et al., 1996). NELL2 mRNA is predominantly expressed in the central nervous system throughout the pre-natal stages, and its expression gradually increases during embryonic development. In the adult rat brain, high levels of NELL2 mRNA are detected in the taenia tecta, piriform cortex, hippocampus, dentate gyrus, cerebellar cortex, ambiguous nucleus, and inferior olivary nucleus (Kim et al., 2002; Oyasu et al., 2000). In addition, NELL2 is an estrogen-induced gene in the adult female rat hypothalamus and is involved in the estrogen-dependent organization of the sexually dimorphic nucleus in the preoptic area (Jeong et al., 2008). These results strongly suggest that NELL2 may be involved in important roles in brain functions.
Although NELL2 was identified as a binding protein of a protein kinase C isoform (i.e., PKCβ1) using a yeast two-hybrid assay, suggesting that NELL2 is a cytoplasmic protein (Kuroda and Tanizawa, 1999), most subsequent papers about NELL2 have strongly suggested that this protein is a secreted glycoprotein (Kim et al., 2010; Nelson et al., 2004). NELL2 genes encode peptides of approximately 90 kDa that are highly glycosylated, resulting in a total monomeric size of approximately 130 kDa in COS-7 cells (Nelson et al., 2004). Secreted NELL2 has been suggested to have a putative function as a trophic factor (Matsuhashi et al., 1995; Watanabe et al., 1996). A study of the effect of recombinant rat NELL2 proteins on primary cultured neurons provided direct evidence that secreted NELL2 promotes the survival of primary neurons by decreasing mitogen-activated protein kinase activities (Aihara et al., 2003). Secreted NELL2 acts in a paracrine manner to stimulate the mitogenesis of adjacent cells within the nascent chick dorsal root ganglia (Nelson et al., 2004), and ectopic expression of NELL2 also promotes differentiation of neuronal progenitor HiB5 cells (Kim et al., 2010). Although NELL2 has been suggested to be a secreted glycoprotein in previous papers, the secretion mechanism of this protein is still unclear.
In the present study, we examined the intracellular localization and movement of NELL2 using C-terminal EGFP-tagged NELL2 (NELL2-EGFP) in living HiB5 cells, a hippocampal progenitor cell line. Analysis of time-lapse images of cells expressing NELL2-EGFP revealed that NELL2 exhibited fast and predominantly anterograde movement with intermittent retrograde movement in living HiB5 cells. In addition, we showed that NELL2 was transported in a microtubule-dependent manner and that the N-terminal 29 amino acids of NELL2 were also critical for the secretion of NELL2.
MATERIALS AND METHODS
Construction of plasmids and chemicals
To construct expression vectors for NELL2 and amyloid precursor protein (APP), previously obtained rat full-length NELL2 cDNA (GenBank accession number: AY089719) and human full-length APP cDNA (GenBank accession number: NM_000484) were amplified and inserted into several expression vectors using the Gateway Cloning System as previously described (Hwang et al., 2007). Two expression vectors of NELL2 (non-tagged, pDS_NELL2; C-terminal EGFP-tagged, pDS_NELL2-EGFP) were constructed. The N-terminal 29-amino acid peptide (1–29 amino acids of NELL2) and a deletion construct in which this region was omitted (30–816 amino acids of NELL2) were also constructed (pDS_Sig-EGFP, Sig; pDS_ΔSig-NELL2-EGFP, ΔSig). Nocodazole and cytochalasin D were purchased from Sigma-Aldrich (Sigma-Aldrich, USA).
Cell culture and transfection
The rat hippocampal progenitor cell line, HiB5, was grown as previously reported (Park et al., 2012). Briefly, the cells were maintained at 33°C (their permissive temperature) in DMEM (GIBCO BRL, USA) containing penicillin G (100 U/ml; GIBCO BRL), streptomycin sulfate (100 μg/ml; GIBCO BRL), and 10% fetal bovine serum (GIBCO BRL). When cells reached 85% confluency, they were transfected with one of the expression vectors (pDS_NELL2, pDS_NELL2-EGFP, pDS_Sig-EGFP [Sig], or pDS_ΔSig-NELL2-EGFP [ΔSig]) using a MP-100 microporator (Digital Bio, Korea) according to the manufacturer’s instructions.
Western blotting
Fifty micrograms of the extracted proteins from transfected cells were used for Western blotting as described (Hwang et al., 2007). Hybridization was performed using anti-green fluorescent protein (GFP) antibody [Santa Cruz Biotechnology Inc. (Germany), 1:1000] and anti-NELL2 antibody (1:500, see Ha et al., 2008 for the generation and verification of the antibody). The epitope of the anti-NELL2 antibody is amino acids 273–588 of the rat NELL2 protein. To determine release of NELL2, transfected cells were incubated for 2 h in serum-free DMEM. Subsequently, culture medium and cell lysates were obtained, and proteins were precipitated with 10% trichloroacetic acid. Western blotting was performed as described above using the anti-GFP antibody. Subcellular fractionation was performed as previously described (Stern et al., 2007) with HEK293T cells expressing EGFP, APP-EGFP, or NELL2-EGFP.
Immunocytochemistry
HiB5 cells expressing NELL2 were grown on coverslips (25 mm), washed with phosphate-buffered saline (PBS), and fixed with 4% (w/v) paraformaldehyde in PBS at room temperature for 30 min. The cells were washed again with PBS and permeabilized by incubating in PBS containing 0.25% (v/v) Triton X-100, 5% (v/v) normal goat serum, and 5% (w/v) skim milk for 30 min at room temperature. The anti-NELL2 antibody was added at a concentration of 0.4 μg/ml and allowed to react overnight at 4°C. For actin and tubulin staining, β-actin (Sigma; 1:500) and α-tubulin (Sigma; 1:1000) were used.
Imaging analysis
Transfected HiB5 cells on coverslips (25 mm diameter) were placed in a perfusion chamber at room temperature and maintained in DMEM. Intracellular localization of NELL2-EGFP was analyzed with a confocal microscope (Olympus Fluoview FV 1,000) with a 60× /1.4 numerical aperture Plan-Apochromat objective. When needed, subcellular localization vectors such as pECFP-ER, pECFP-Golgi, and pDsRed-Mito (Clontech Laboratories, USA) were cotransfected with NELL2-EGFP. Time-lapse images of NELL2-EGFP in motion were captured. One image at a resolution of 512 by 512 pixels was taken every 1.67 s. The fluorescence data from single cells were stored and processed with Fluoview 1000 software.
RESULTS
Expression and localization of EGFP-tagged NELL2 in HiB5 cells
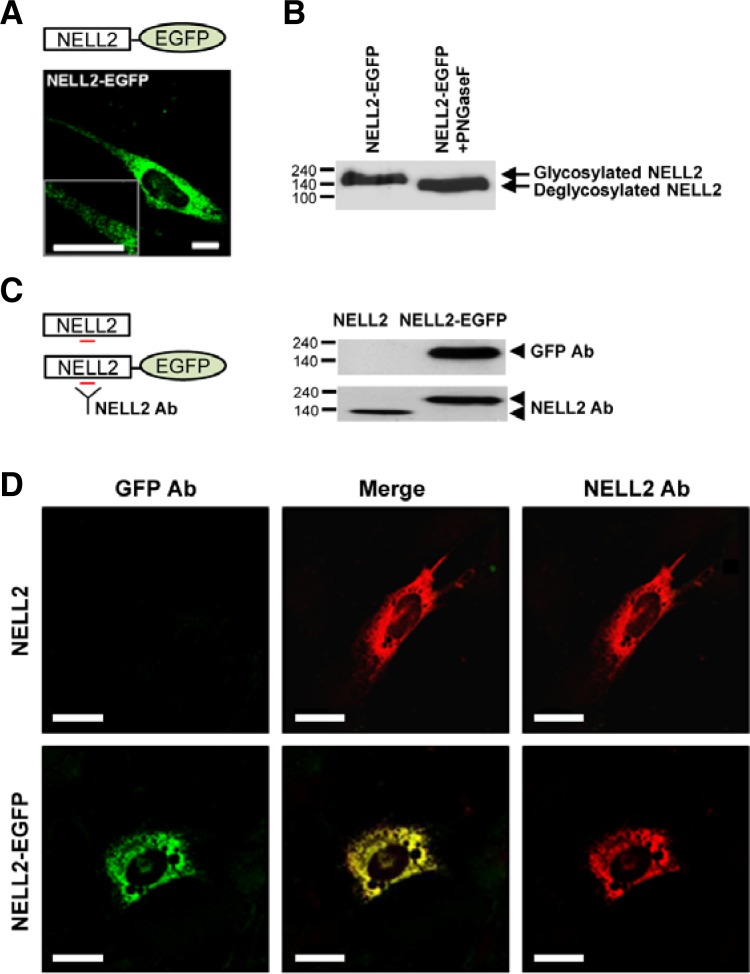
To visualize the localization of rat NELL2 in HiB5 cells, we generated a construct containing EGFP-tagged full-length rat NELL2. NELL2-EGFP was expressed in distinct subcellular structures of HiB5 cells when the EGFP was tagged to the C-terminal end of NELL2, and Western blotting revealed a molecular weight of approximately 167 kDa (∼140 kDa + 27 kDa EGFP). This size was reduced by treatment with N-Glycosidase F (Fig. 1B), suggesting that NELL2 was glycosylated with N-linked carbohydrate moieties, as has been previously reported (Nelson et al., 2004). Treatment with tunicamycin, an inhibitor of glycosylation, also reduced the sizes of NELL2 and NELL2-EGFP (Supplementary Fig. 1A). These data imply that NELL2-EGFP can be used to study the functions of NELL2 protein. We also confirmed the specific size of non-tagged NELL2 and NELL2-EGFP with Western blotting using a NELL2 antibody (Fig. 1C, left). The NELL2 antibody detected both NELL2 and NELL2-EGFP (Fig. 1C, right).
Fig. 1.
Characterization of NELL2 in HiB5 cells. (A) HiB5 cell expressing NELL2-EGFP was observed by confocal microscopy. Scale bar = 10 μm. A schematic of NELL2-EGFP is located in the upper of (A). (B) Analysis of glycosylation of the NELL2-EGFP protein with N-glycosidase F (PNGase F). The lysates from HiB5 cells expressing NELL2-EGFP were incubated without or with PNGase F 1 h at 37°C. Proteins were then analyzed by Western blot analysis with GFP antibody. The protein size of NELL2-EGFP was decreased by treatment with PNGase F. The sizes of the molecular weight markers (in thousands) are indicated on the left. (C) A schematic of NELL2-EGFP illustrates the binding of NELL2 antibody to the antigenic region (left). HiB5 cells were harvested 48 h after the transfection of expression vectors. NELL2 and NELL2-EGFP were analyzed by immunoblotting with GFP antibody or NELL2 antibody, respectively (right). The sizes of the molecular weight markers (in thousands) are indicated on the left. (D) HiB5 cells expressing either NELL2 or NELL2-GFP were stained with NELL2 antibody. The two proteins showed similar distribution patterns within HiB5 cells. Scale bar = 20 μm.
We next determined whether the subcellular distribution of NELL2 in HiB5 cells was affected by the EGFP tag. NELL2-EGFP was distributed in an expression pattern similar to the distribution pattern of untagged exogenous NELL2 (Fig. 1D). Furthermore, when NELL2 was coexpressed with pECFP-ER [endoplasmic reticulum (ER)], pECFP-Golgi (Golgi), and pDsRed-Mito (mitochondria) in HiB5 cells, NELL2-EGFP was densely colocalized in the Golgi apparatus and ER but not in the mitochondria (Supplementary Fig. 1B). Interestingly, NELL2-EGFP appeared as fluorescent particles of various sizes and rounded shapes at higher magnification. These particles moved rapidly from the Golgi apparatus to the plasma membrane (see Supplementary Video 1). This indicates that NELL2 particles were transported to the membrane via the trans-Golgi network.
Bidirectional movements of NELL2-EGFP vesicles in living HiB5 cells
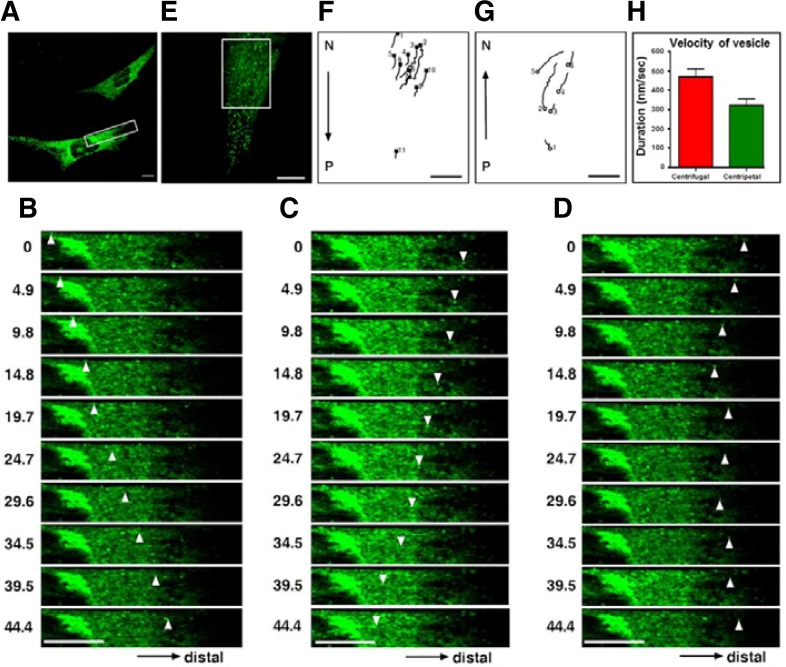
As described above, NELL2 was localized in regions involved in the secretory pathway (ER, Golgi apparatus, and moving fluorescent particles). We assumed that these moving fluorescent particles were vesicles containing NELL2 and focused on the characterization of NELL2 vesicle movement in living HiB5 cells. Time-lapse images of NELL2-transfected HiB5 cells showed that NELL2 movements occurred predominantly in the cytoplasm (Fig. 2A); although, NELL2 movements were also detected in the perinuclear region. The overall direction of NELL2 movements (70.1% of vesicles) was anterograde (moving away from the Golgi); although, retrograde movements were observed in 28.7% of vesicles (n = 100; Figs. 2B and 2C). Some NELL2 vesicles changed direction multiple times during observation (Fig. 2D). Figures 2F and 2G show that the tracks of some NELL2 vesicles exhibited both centrifugal (F) and centripetal (G) movement within the boxed area in Fig. 2E. The velocity of NELL2-EGFP-containing punctate structures was 0.454 ± 0.143 μm/s for centrifugal movements (anterograde direction) and 0.302 ± 0.088 μm/s for centripetal movements (retrograde direction) (Fig. 2H). Importantly, the fluorescence intensity of moving NELL2 vesicles did not change significantly (96%, n = 40) during the period of either centrifugal or centripetal movement. This phenomenon suggests that NELL2 is inserted into the vesicle membrane.
Fig. 2.
Bidirectional movements of NELL2 in HiB5 cells. (A) The typical expression pattern of NELL2-EGFP in HiB5 cells. Successive video stills taken at 4.90 s intervals of typical anterograde (B) and retrograde (C) movements (white arrow head). Some NELL2 vesicles underwent multiple changes in direction (D) within the boxed area in indicated in (A). Diagrams of the paths of some of the anterograde (F) and retrograde (G). NELL2 movements over a one minute period from the cell shown in (E). Starting points are indicated by either a closed square (anterograde) or an open circle (retrograde). The path diagrams for this, and some subsequent figures, are reoriented relative to the image of the cell, so that up is towards the nucleus (N) and down is towards the periphery (P). Scale bar = 20 μm. (H) The average velocity of anterograde (red) and retrograde (green) vesicle movement in living HiB5 cells.
NELL2 is not localized at the plasma membrane
NELL2 vesicles were rapidly transported to the peripheral region of HiB5 cells, implying that NELL2 may act as a secreted protein or integral membrane protein. Although previous studies suggested that NELL2 is secreted into the culture medium (Hwang et al., 2007; Kim et al., 2010; Nelson et al., 2004), we could not exclude the possibility that NELL2 may act as an integral membrane protein because NELL2 was predicted to be a single transmembrane helix by SOSUI software from ExPASy Proteomics. To examine whether NELL2 localized to the plasma membrane, NELL2-EGFP-transfected HiB5 cells were fixed and either permeabilized with Triton X-100 or left untreated. Both groups of cells were then stained with either GFP antibody (GFP Ab), which recognizes the EGFP tag, or rabbit polyclonal NELL2 antibody (NELL2 Ab). Although Triton X-100-pretreated cells were immunoreactive to both GFP Ab and NELL2 Ab, non-permeabilized cells were not stained with GFP Ab or NELL2 Ab (Supplementary Figs. 2A and 2B). A fractionation experiment also showed that NELL2-EGFP and control EGFP were not localized in the membrane fraction, whereas APP-EGFP, a type I membrane protein, was localized in the membrane fraction (Supplementary Fig. 2C). This result suggested that NELL2 exists as a secreted protein rather than an integral membrane protein and can be secreted into the medium.
Microtubule-dependent transport of NELL2-EGFP vesicles
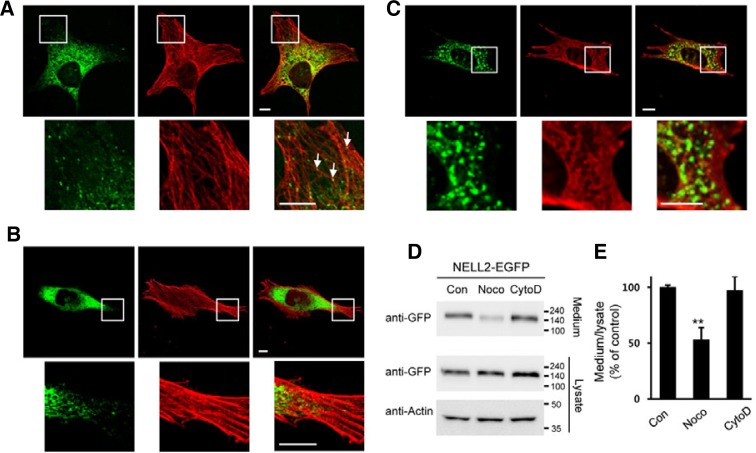
Microtubules and actin filaments significantly contribute to directional mobility in neuronal axons (Barkus et al., 2008). NELL2 associates with microtubule-actin crosslinking factor 1 (Macf1) in retinal ganglion cells (Munemasa et al., 2012). Based on these previous reports and our fluorescent imaging of NELL2-EGFP trafficking (Fig. 2), we hypothesized the possible interaction of NELL2 vesicles with cytoskeletal elements. To examine this possibility, double labeling of NELL2 and microtubules (α-tubulin) or microfilaments (β-actin) was performed. NELL2-EGFP and α-tubulin staining patterns were similar, and higher-magnification images more clearly revealed the colocalization of NELL2 vesicles with α-tubulin (Fig. 3A). In contrast to our findings with α-tubulin, NELL2 vesicles were not colocalized with actin filaments (Fig. 3B). When NELL2-expressing HiB5 cells were treated with 2 μM nocodazole (an inhibitor of microtubule polymerization), the microtubules were almost completely depolymerized, resulting in a partially dispersed Golgi apparatus. Two major changes occurred following nocodazole treatment. First, the NELL2 vesicles showed very little directed movement (data not shown). Second, vesicles tended to become aggregated and lost their typical expression pattern (Fig. 3C). Next, to examine the effects of cytoskeletal elements on the secretion of NELL2, culture media of NELL2-EGFP-transfected HiB5 cells were treated with inhibitors of cytoskeletal elements, harvested, and subjected to Western blot analysis using anti-GFP antibody. The secretion of NELL2 was significantly reduced by treatment with 2 μM nocodazole for 4 h (Figs. 3D and 3E). However, treatment with 2 μM cytochalasin D (a potent inhibitor of actin polymerization) did not affect secretion of this protein. These results clearly show that microtubules, but probably not actin, are necessary for efficient movement and secretion of NELL2 proteins.
Fig. 3.
NELL2 colocalized with tubulin but not actin in HiB5 cells. HiB5 cells were transiently transfected with NELL2-EGFP (green) and immunostained with α-tubulin or β-actin (red). Scale bar = 20 μm. (A) Magnification of the boxed area in the upper panels reveals that NELL2 vesicles localized with α-tubulin. Arrows indicates colocalization between microtubules and NELL2-EGFP vesicles. (B) Magnification of the boxed area in the upper panels revealed that NELL2 vesicles were not colocalized with β-actin. (C) Treatment of nocodazole triggered aggregation of NELL2 vesicles (green) and loss of their typical expression pattern (α-tubulin; red). (D) Western blot analysis of NELL2 protein in the medium and lysate of sNELL2-EGFP transfected COS-7 cells. Expression level of actin was used as loading control. The sizes of the molecular weight markers (in thousands) are indicated on the left. (E) Treatment of nocodazole, but not cytochalasin D, dramatically reduced secretion of sNELL2-EGFP (** P < 0.01, ANOVA).
The N-terminal 29 amino acids of NELL2 are responsible for secretion
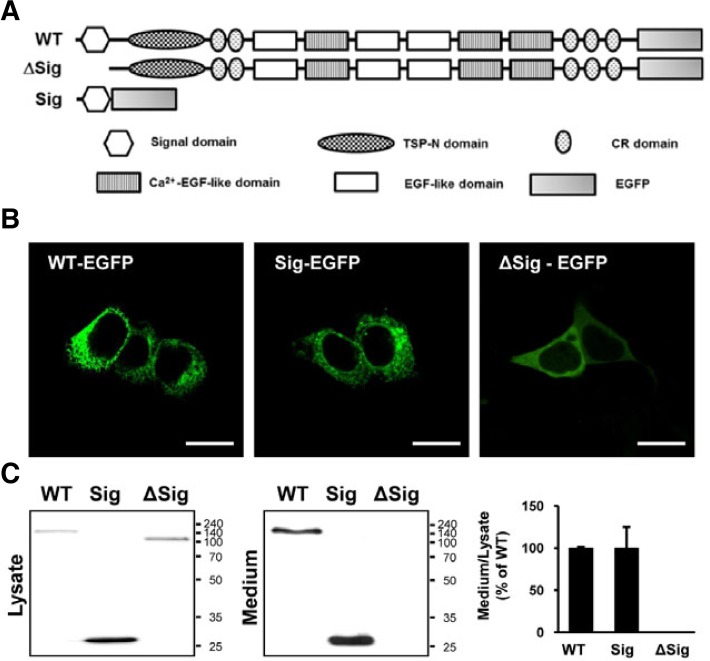
Although previous studies deduced that NELL2 contains several functional domains in its amino acid sequence (Kuroda et al., 1999; Watanabe et al., 1996) (Fig. 4A), which domain is critical for NELL2 secretion has not been characterized. Based on the secretion level of various domain-deleted NELL2 mutants, we found that the N-terminal signal domain is critical for NELL2 secretion (data not shown). As shown in Fig. 4B, the N-terminal 29 amino acids (deduced signal domain of 21 amino acids + 8 linker amino acids) of NELL2 directly fused with EGFP (Sig) showed expression patterns similar to that of wild-type NELL2-EGFP in HiB5 cells, whereas the signal domain-deleted NELL2 (ΔSig) was diffusely expressed in the cytoplasm of the cells. In addition, Sig protein and wild-type NELL2, but not Δ Sig, were detected in conditioned medium (Fig. 4C). These data suggest that the N-terminal signal domain of NELL2 is important for intracellular expression and secretion of NELL2.
Fig. 4.
The N-terminal 29 amino acids of NELL2 are critical for secretion of NELL2. (A) The three C-terminal EGFP-tagged constructs used in this study. Wild-type NELL2 contains the following domains: N-terminal thrombospondin-1-like domain (TSP-N), five von Willebrand factor C domains (CR), and EGF-like domains. Two NELL2 mutants were also constructed. One has only the putative signal peptide domain (Sig) and the other lack the putative signal peptide (ΔSig). (B) HiB5 cells expressing NELL2-EGFP (WT), Sig and Δ Sig were observed by confocal microscope. Scale bar = 20 μm. (C) HiB5 cells were transiently transfected with WT, Sig and ΔSig. The cell lysates (left panel) and culture media (middle panel) of these transfected cells were immunoblotted with GFP antibody. The histogram shows percentages of secretion level of NELL2 mutants comparing to that of wild-type NELL2 (right panel). Secretion level of each protein was analyzed from the ratio of amounts in the medium to that in the lysate. Notice just putative signal peptide was secreted to medium such as NELL2 full length secretion.
DISCUSSION
Several functions of NELL2 in the brain were reported in previous papers. NELL2 acts on neuronal cells to promote differentiation, proliferation (Kim et al., 2010; Nelson et al., 2004), and survival through mitogen-activated protein kinases (Aihara et al., 2003). NELL2 also supports the survival of retinal ganglion cells after optic nerve injury (Munemasa et al., 2012), and NELL2 is involved in the formation of the sexually dimorphic nucleus of the preoptic area (Jeong et al., 2008). NELL2-deficient mice may have hippocampal neurons with altered differrentiation and/or signal transduction properties, which ultimately lead to long-term potentiation enhancement and spatial memory impairment (Matsuyama et al., 2004; 2005). Despite these important roles for NELL2 in brain function, the intracellular transport and secretion mechanism of NELL2 have not yet been determined.
Here, we investigated the trafficking and secretion mechanisms of NELL2 using time-lapse imaging of hippocampal progenitor cells. Our results showed that the addition of an EGFP tag to the C-terminus of NELL2 did not affect the transport and post-translational processing of NELL2. Consequently, NELL2-EGFP was secreted into the culture medium. The molecular size was reduced after deglycosylation with N-glycosidase F treatment of the cell extract and medium. However, N-terminal EGFP-tagged NELL2 (EGFP-NELL2) did not exhibit an expression pattern typical of NELL2, which is expressed in the trans-Golgi network and shows an increased molecular weight. These results are consistent with previous studies that predicted structural features such as a cleavable signal peptide and N-glycosylation of the protein (Nelson et al., 2004; Oyasu et al., 2000). One of the most prominent characteristics of NELL2 is its speed and anterograde movement. We also demonstrated that NELL2 colocalized with α-tubulin, and the depolymerization of microtubules resulted in reduction of vesicle speed and aggregation of punctate structures in HiB5 cells.
Interestingly, the instantaneous velocity of NELL2-EGFP-containing punctate structures was 0.454 ± 0.143 μm/s for anterograde movement and 0.302 ± 0.088 μm/s for retrograde movement in HiB5 cells (Fig. 3H). These results suggest that the protein(s) that regulate the velocity of NELL2 movement may exist in these cells. The velocity of NELL2 movement is comparable to that of the kinesin motor family that is expressed in axons (Goldstein and Yang, 2000). For example, a kinesin motor protein, KIF1A, moves anterogradely along neurites with an average velocity of 1.0 μm/s and retrogradely at 0.72 μm/s in cultured hippocampal neurons (Lee et al., 2003). KIF2 and KIF3 move with a velocity ranging from 0.3–0.5 μm/s (Noda et al., 1995; Yamazaki et al., 1995). Moreover, approximately 70% of the NELL2 vesicles in motion were anterogradely and rapidly transported, similar to most members of the kinesin family (∼80%) along the microtubules (Goldstein and Yang, 2000; Lee et al., 2003). Thus, NELL2 vesicles may be associated with kinesin motor family members or an accessory motor protein, such as kinesin-associated protein 3, KAP3.
Movement of NELL2 vesicles was composed of rapid “run-and-pause” episodes that are characteristic of the movement of microtubule-dependent vesicles (Vugmeyster et al., 1998). Figure 3 shows that NELL2 was colocalized with α-tubulin in HiB5 cells, and the depolymerization of microtubules resulted in reduction in vesicle movement and aggregation of vesicles. These results suggested that NELL2 vesicles are mainly transported along microtubules. In addition, a recent study showed that NELL2 interacts with Macf1 in retinal ganglion cells (Munemasa et al., 2012). Because Macf1 may be involved in the translocation of proteins along axons, and its associated complex (composed of Apc, β-catenin, and Gsk3β) moves from the cytoplasm to the cell membrane (Munemasa et al., 2012), NELL2 transport in HiB5 cells may also be regulated by these proteins. Further investigations are needed to resolve the detailed mechanisms of NELL2 transport.
We also demonstrated that NELL2 did not accumulate in the periphery of HiB5 cells, and surface staining using a NELL2 antibody showed that the protein rarely remained on the surface. These results suggest that the majority of NELL2 is secreted into the medium, or that NELL2 is rapidly degraded at its destination. Figure 4 shows that NELL2 was clearly secreted into the medium but not degraded. Because NELL2 with the N-terminal 29 amino acids deleted (ΔSig) was not secreted into the medium (Fig. 4B), this region is critical for the secretion of NELL2. In addition, we previously reported the existence of a splice variant of NELL2, cytosolic NELL2 (cNELL2), in which the third exon (corresponding to amino acids 20–62) is spliced out, causing localization to the cytosol and involvement in PKCβ-mediated intracellular signaling (Hwang et al., 2007). These data indicate that the N-terminal region of NELL2 likely contains a signal domain that is important for proper localization and secretion of the protein.
In the present study, our results clearly show that NELL2 proteins were localized in distinct subcellular structures (ER, Golgi apparatus, and secretable vesicles) and were secreted into the medium. NELL2 moved bidirectionally along microtubules, and the N-terminal signal domain was important for the secretion and cellular localization. These data contribute to the understanding of the mechanisms of neuronal differentiation, as well as the factors that influence neuronal survival.
Supplementary Material
Acknowledgments
This research was supported by Basic Research Promotion Fund (NRF-2008-313-C00758) for J.Y. Park and Basic Science Research Programs (NRF-2010-0005798) for E.M. Hwang and (NRF-2010-0022375) for C.M. Ha funded by the National Research Foundation of Korea (NRF) and also supported by the Establishment and Operation of Korea Brain Research Institute (KBRI) funded by the Ministry of Science, ICT and Future Planning (2031-415) for C.M. Ha.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Aihara K, Kuroda S, Kanayama N, Matsuyama S, Tanizawa K, Horie M. A neuron-specific EGF family protein, NELL2, promotes survival of neurons through mitogen-activated protein kinases. Brain Res Mol Brain Res. 2003;116:86–93. doi: 10.1016/s0169-328x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- Barkus RV, Klyachko O, Horiuchi D, Dickson BJ, Saxton WM. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol. Biol. Cell. 2008;19:274–283. doi: 10.1091/mbc.E07-03-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LS, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- Ha CM, Choi J, Choi EJ, Costa ME, Lee BJ, Ojeda SR. NELL2, a neuron-specific EGF-like protein, is selectively expressed in glutamatergic neurons and contributes to the glutamatergic control of GnRH neurons at puberty. Neuroendocrinology. 2008;88:199–211. doi: 10.1159/000139579. [DOI] [PubMed] [Google Scholar]
- Hwang EM, Kim DG, Lee BJ, Choi J, Kim E, Park N, Kang D, Han J, Choi WS, Hong SG, et al. Alternative splicing generates a novel non-secretable cytosolic isoform of NELL2. Biochem Biophys Res Commun. 2007;353:805–811. doi: 10.1016/j.bbrc.2006.12.115. [DOI] [PubMed] [Google Scholar]
- Jeong JK, Ryu BJ, Choi J, Kim DH, Choi EJ, Park JW, Park JJ, Lee BJ. NELL2 participates in formation of the sexually dimorphic nucleus of the pre-optic area in rats. J Neurochem. 2008;106:1604–1613. doi: 10.1111/j.1471-4159.2008.05505.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Ha CM, Choi J, Choi EJ, Jeon J, Kim C, Park SK, Kang SS, Kim K, Lee BJ. Ontogeny and the possible function of a novel epidermal growth factorlike repeat domain-containing protein, NELL2, in the rat brain. J Neurochem. 2002;83:1389–1400. doi: 10.1046/j.1471-4159.2002.01245.x. [DOI] [PubMed] [Google Scholar]
- Kim DG, Hwang EM, Yoo JC, Kim E, Park N, Rhee S, Ha CM, Hong SG, Park JY. Identification and characterization of a truncated isoform of NELL2. Biochem Biophys Res Commun. 2010;391:529–534. doi: 10.1016/j.bbrc.2009.11.092. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Tanizawa K. Involvement of epidermal growth factor-like domain of NELL proteins in the novel protein-protein interaction with protein kinase C. Biochem Biophys Res Commun. 1999;265:752–757. doi: 10.1006/bbrc.1999.1753. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Oyasu M, Kawakami M, Kanayama N, Tanizawa K, Saito N, Abe T, Matsuhashi S, Ting K. Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun. 1999;265:151–160. doi: 10.1006/bbrc.1999.1638. [DOI] [PubMed] [Google Scholar]
- Lee JR, Shin H, Ko J, Choi J, Lee H, Kim E. Characterization of the movement of the kinesin motor KIF1A in living cultured neurons. J Biol Chem. 2003;278:2624–2629. doi: 10.1074/jbc.M211152200. [DOI] [PubMed] [Google Scholar]
- Luce MJ, Burrows PD. The neuronal EGF-related genes NELL1 and NELL2 are expressed in hemopoietic cells and developmentally regulated in the B lineage. Gene. 1999;231:121–126. doi: 10.1016/s0378-1119(99)00093-1. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S, Noji S, Koyama E, Myokai F, Ohuchi H, Taniguchi S, Hori K. New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev Dyn. 1995;203:212–222. doi: 10.1002/aja.1002030209. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Aihara K, Nishino N, Takeda S, Tanizawa K, Kuroda S, Horie M. Enhanced long-term potentiation in vivo in dentate gyrus of NELL2-deficient mice. Neuroreport. 2004;15:417–420. doi: 10.1097/00001756-200403010-00007. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Doe N, Kurihara N, Tanizawa K, Kuroda S, Iso H, Horie M. Spatial learning of mice lacking a neuron-specific epidermal growth factor family protein, NELL2. J Pharmacol Sci. 2005;98:239–243. doi: 10.1254/jphs.fp0050211. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, Chang CS, Kwong JM, Kyung H, Kitaoka Y, Caprioli J, Piri N. The neuronal EGF-related gene Nell2 interacts with Macf1 and supports survival of retinal ganglion cells after optic nerve injury. PLoS One. 2012;7:e34810. doi: 10.1371/journal.pone.0034810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BR, Claes K, Todd V, Chaverra M, Lefcort F. NELL2 promotes motor and sensory neuron differentiation and stimulates mitogenesis in DRG in vivo. Dev Biol. 2004;270:322–335. doi: 10.1016/j.ydbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Noda Y, Sato-Yoshitake R, Kondo S, Nangaku M, Hirokawa N. KIF2 is a new microtubule-based anterograde motor that transports membranous organelles distinct from those carried by kinesin heavy chain or KIF3A/B. J Cell Biol. 1995;129:157–167. doi: 10.1083/jcb.129.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyasu M, Kuroda S, Nakashita M, Fujimiya M, Kikkawa U, Saito N. Immunocytochemical localization of a neuron-specific thrombospondin-1-like protein, NELL2: light and electron microscopic studies in the rat brain. Mol Brain Res. 2000;76:151–160. doi: 10.1016/s0169-328x(99)00342-3. [DOI] [PubMed] [Google Scholar]
- Park N, Yoo JC, Ryu J, Hong SG, Hwang EM, Park JY. Copine1 enhances neuronal differentiation of the hippocampal protenitor HiB5 cells. Mol. Cells. 2012;34:549–554. doi: 10.1007/s10059-012-0235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern KA, Smit GD, Place TL, Winistorfer S, Piper RC, Lill NL. Epidermal growth factor receptor fate is controlled by Hrs tyrosine phosphorylation sites that regulate Hrs degradation. Mol. Cell Biol. 2007;27:888–898. doi: 10.1128/MCB.02356-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vugmeyster Y, Berliner E, Gelles J. Release of isolated single kinesin molecules form microtubules. Biochemistry. 1998;37:747–757. doi: 10.1021/bi971534o. [DOI] [PubMed] [Google Scholar]
- Watanabe TK, Katagiri T, Suzuki M, Shimizu F, Fujiwara T, Kanemoto N, Nakamura Y, Hirai Y, Maekawa H, Takahashi E. Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. 1996;38:273–276. doi: 10.1006/geno.1996.0628. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Nakata T, Okada Y, Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol. 1995;130:1387–1399. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.