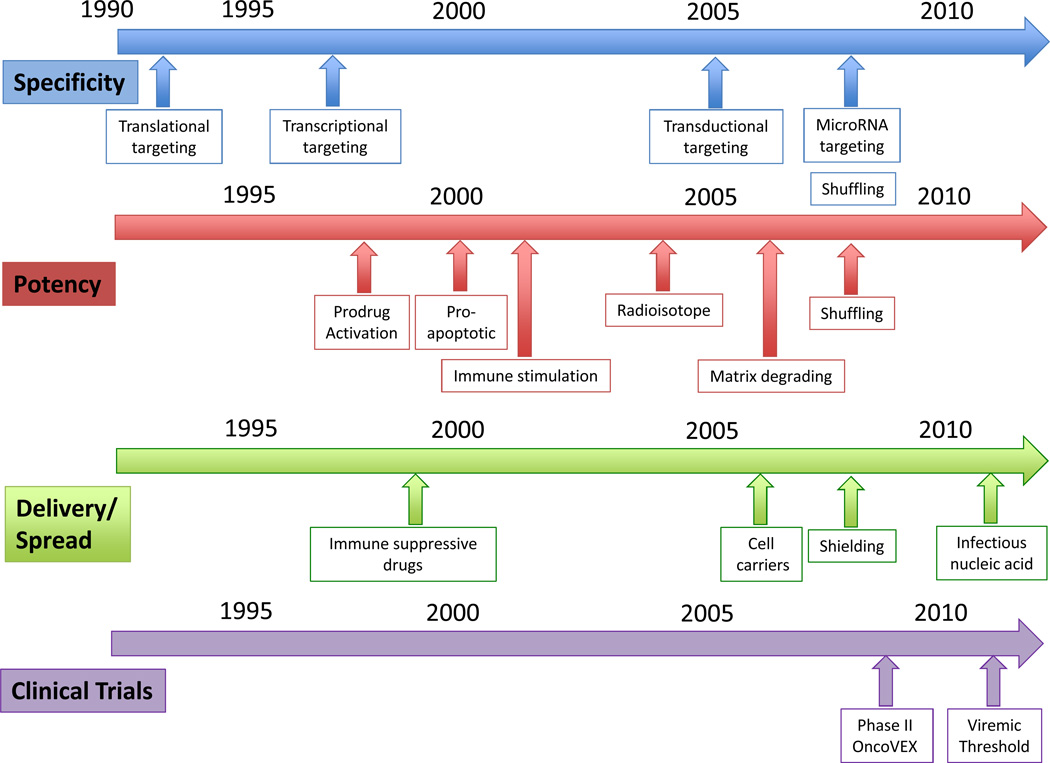
Figure 3. A timeline of milestones in the development of oncolytic virotherapy to improve virus specificity, potency, delivery and spread.
SPECIFICITY: (1) Translational targeting: 1991, Engineering of a replication-competent HSV attenuated for neurovirulence for glioma treatment10. (2) Transcriptional targeting: 1997, targeting of HSV using albumin promoter/enhancer for hepatoma cells161 and Ad using Prostate specific antigen (PSA) promoter for prostate cancer cells162, (3) Transductional targeting: 2005, targeting entry and cytopathic effects of oncolytic measles virus by display of single-chain antibody on the virus attachment protein, (4) MicroRNA targeting: 2008, to control unwanted toxicity of picornarvirus131 and vesicular stomatitis virus132 while retaining antitumor activity, (5) DNA shuffling, 2008, Mixing a pool adenoviral serotypes and passaging the pools under conditions that invite recombination between serotypes to generate tumor selective virus163.
POTENCY: (1) Prodrug activation: 1998, an oncolytic adenovirus expressing cytosine deaminase and HSV-Tk designed to work in combination with 5-FC and Ganciclovir, (2) Proapoptotic genes: 2000, introduction of the adenovirus death protein (ADP) into an oncolytic adenovirus to enhance its cytotoxicity164, (3) Immune stimulation: 2001, oncolytic HSV encoding IL-12 and GM-CSF to recruit T lymphocyte-mediated antitumor immune response165, (4) Radioisotope: 2004, an oncolytic measles virus encoding the human sodium iodide symporter (NIS) which concentrates beta-emitting (radiovirotherapy) and gamma-emitting isotopes (imaging)33, (5) 2006, Matrix degrading proteins: adenovirus encoding relaxin protein to enhance virus intratumoral spread166, (6) Shuffling: 2008, Mixing a pool adenoviral serotypes and passaging the pools under conditions that invite recombination between serotypes to generate more potent adenovirus ColoAd1 163.
DELIVERY & SPREAD: (1) Immune suppressive drugs: 1999, Addition of cyclophosphamide to combat and innate and adaptive antiviral immunity to enhance intratumoral spread of HSV, (2) Cell carriers: 2006, use of cytokine induced killer cells to deliver oncolytic vaccinia virus to tumor, resulting in synergistic antitumor activity167, (3) Shielding: 2008, Polymer coating and retargeting of oncolytic adenovirus for ovarian cancer to enhance viral pharmacokinetics40, (4) Infectious Nucleic Acid: 2011, delivery of oncolytic picornarvirus using infectious nucleic acid (RNA) to successfully achieve sustained viremia and tumor regression.
CLINICAL TRIALS: (1) Activity: 2009, Phase II trial with intralesional injection of oncolytic HSV, OncoVEX (talimogene laherparepvec ), in melanoma patients. 26% complete response (8 out of 50), with durability in both injected and uninjected lesions including visceral sites19. Undergoing Phase III evaluation. (2) Viremic Threshold: 2011, Intravenous delivery of JX-594, oncolytic vaccinia virus, in patients with metastatic tumor, demonstrating the need for a viremic threshold to be reached for efficient virus delivery to tumors26.