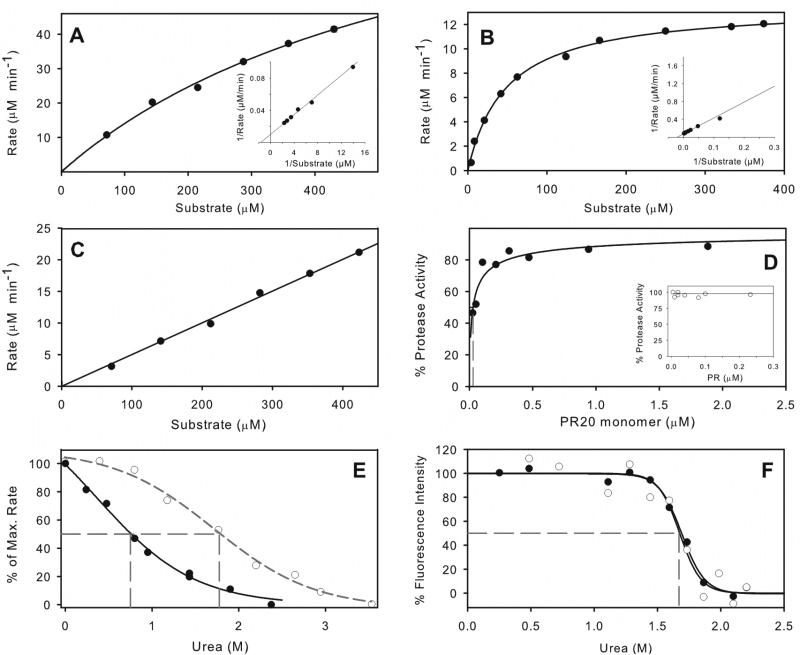
Figure 3.
Kinetic properties of PR20 and comparison with PR. Michaelis-Menten kinetics for hydrolysis of substrate IV by 0.5 μM PR20 in 50 mM sodium acetate buffer, pH 5, containing 250 mM sodium chloride (A), and its hydrolysis under the same conditions31 catalyzed by 0.078 μM PR (B). Kinetic parameters for PR20 are kcat ≥196 min−1 and Km ≥533 μM. For PR, kcat = 174 ± 3 min−1 and Km = 48.2 ± 3.1 μM. Solid lines are based on curve fitting of the Michaelis-Menten equation to the data. Lineweaver-Burke plots are shown as insets. (C) shows the linear dependence of reaction rate for PR20 hydrolysis on substrate concentration, resulting from an increased Km in the presence of 0.8 M urea under the same conditions as (A). (D) Dependence of catalytic activity on the concentration of PR20 in the same buffer gives a Kd of 29 ± 10 nM (dashed gray line). For comparison the inset shows that Kd for PR is < 5 nM under similar conditions20 (E) Effect of increasing urea concentration on hydrolysis of substrate IV by PR20 (solid symbols) and by PR20 (open symbols). (F) Effect of urea concentration on the intrinsic fluorescence of PR20 in 50 mM sodium acetate buffer, pH 5. Solid circles, data in the absence of inhibitor; open circles, in the presence of 2 μM DRV. The urea concentrations at which 50% of the maximum effect (UC50) is observed are indicated by dashed gray lines in (E) and (F).