Abstract
Human islet transplantation is an effective and promising therapy for Type I diabetes. However, long-term insulin independence is both difficult to achieve and inconsistent. De novo or early administration of incretin-based drugs is being explored for improving islet engraftment. In addition to its glucose-dependent insulinotropic effects, incretins also lower postprandial glucose excursion by inhibiting glucagon secretion, delaying gastric emptying, and can protect beta-cell function. Incretin therapy has so far proven clinically safe and tolerable with little hypoglycemic risk. The present review aims at highlighting the new frontiers in research involving incretins from both in vitro and in vivo animal studies in the field of islet transplant. It also provides an overview of the current clinical status of incretin usage in islet transplantation in the management of Type I diabetes.
Keywords: GLP-1, GLP-1 Receptor, Exendin-4, Liraglutide, DPP IV Inhibitor, Pancreatic Islet of Langerhans and Beta-cells, Human Islet Transplantation, Glycemia, Glucagon-Like Peptide-1, Islet Graft Performance
Introduction
For Type I diabetes mellitus (TIDM) with refractory hypoglycemia, islet transplantation is a promising therapy and has two advantages [1-3]: (i) reduced morbidity and mortality compared to pancreas transplantation and (ii) islet grafts can physiologically regulate glucose homeostasis without hypoglycemic risk. The success of islet transplantation was achieved via the selection of portal vein and liver as a transplant site, improved isolation methods, and steroid-free Edmonton immunosuppressant protocol [1].
The reproducibility of the protocol has been extensively tested worldwide and its effectiveness is proven. However, inconsistent results among centers and variable long-term insulin independence have been observed. At well-established centers, insulin independence at one-year can be achieved in most patients [1, 4]; however, three and five year reports present sobering results, with only between 10-50% remaining insulin independent [5, 6]., Importantly, all patients, even those with partial graft function, have demonstrated a significant improvement in glycemic control, hypoglycemic protection, and reduction of complications [7, 8].
Many confounding factors have been linked to this inconsistency and variation, including low islet quantity/quality, immune attack, immunosuppression toxicity, and a suboptimal microenvironment (hypoxia and higher concentrations of immunosuppressants in the portal system). Attempts to improve transplant outcomes have been intensely investigated for each of the aforementioned aspects [9-11].
There is an increased interest in discovering biological and chemical agents that can be administered to improve graft function, incretin hormones being of particular interest. In this review, we will first discuss the incretins and the underlying beneficial mechanisms for treating diabetes. Then we will review recent developments of incretin both in vitro and in vivo animal studies, focusing on those for islet transplant. Finally, we will discuss their clinical applications for islet transplantation.
1 . Incretins, incretin receptor analogs, and DPP IV inhibitors
The main members of the incretin family are glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). GLP-1 is derived from L-cells of distal intestine, while GIP is released from K-cells of proximal intestine. GLP-1 and GIP are responsible for approximately 50% of postprandial insulin secretion [12]. However, GIP alone does not fully explain incretin’s in vivo effect and has yet to be proven therapeutically effective.
GLP-1 (7-37) and (7-36) are active forms produced from full-length precursors by prohormone convertases 1 and 3 (PC1/3). GLP-1 is quickly degraded by dipeptidyl peptidase IV (DPP IV) resulting in short half-life (1-2 min). Identified in both rodent and human beta-cells, GLP-1R is a type of G-protein couple receptor that activates adenylate cyclase, which in turn affects cAMP-dependent downstream pathways.
GLP-1 stimulates glucose-induced insulin secretion [13] and is critical for controlling fasting plasma glucose, glucose clearance, and gastric emptying [14]. Additionally, GLP-1 can induce insulin biosynthesis by stimulating insulin gene transcription [15], inhibits glucagon mediated by insulin and somatostatin [16], and increases beta-cell mass through an increase in beta-cell proliferation or a decrease in apoptosis [17, 18].
There are two GLP-1 drugs currently approved for clinical use, exendin-4 (also known as exenatide) and liraglutide (also known as Victoza). Approved in 2005, Exendin-4 is a GLP-1R agonist and 39-amino acid peptide sharing 53% homology with full-length GLP-1. Due to its short half-life (3.3–4 hrs), exendin-4 has to be injected twice a day. In 2010 liraglutide, a long-acting GLP-1R agonist, was approved and only needs to be injected once a day. Liraglutide has one amino acid substitution (Arg34Lys) and an attachment of a C-16-free-fatty acid derivative, leading to slower absorption rate, higher binding affinity to albumin, and a half-life of 11–13 hrs. Sitagliptin, the first DPP-IV inhibitor, was approved in 2006. Later on, saxagliptin and vildagliptin were approved as a monotherapy or combinational therapy.
2. Experimental studies of GLP-1 for islet transplantation
2.1 Incretins stimulate islet insulin secretion and improve islet graft function
The Gooszen group was one of first groups to conduct GLP-1 studies for islet transplantation. They showed that GLP-1 resulted in a 2-fold increase in insulin secretion of canine islets in a glucose-dependent manner, starting at 2.5 mM and peaking at 7.5 mM glucose [19]. They also showed that in dog suboptimal mass autologous transplant model, infusion of a physiological dose of GLP-1 at 8.5 mM glucose, which mimics postprandial glycemia, could potentiate insulin secretion by 175% [20].
In 2005, the Weir group studied GLP-1 on islet graft by transplanting GLP-1 pre-treated and non-treated islets in combination with GLP-1 injections for 14 days post-transplant. They indicated that mouse islets cultured with exendin-4 prior to transplantation increased the rate of hyperglycemia reversal, but not to the same degree as freshly isolated islets. Additionally, the mice treated with exendin-4 post transplant did not exhibit any beneficial effect on glucose homeostasis [21]. To clarify the cytoprotective effect of GLP-1 signaling in conditions of glucose toxicity, the Inagaki group compared an isogeneic minimal mass transplant model (150 islets) with a suboptimal model (50 islets) in diabetic mice, showing that exendin-4 contributed to the restoration of normoglycemia in the minimal mass model. However, for suboptimal model group, exendin-4 only prevented beta-cell loss and preserved islet mass initially after transplantation, but could not reverse diabetes. TUNEL staining revealed that exendin-4 reduced the number of apoptotic beta-cells [22]. Two studies from the Tibell and the Juang groups showed similar results using marginal islet transplant models in rodent models [23, 24].
The Tibell group also investigated the role of hypoxia-inducible factor-1 (HIF-1) in mediating the beneficial effects of exendin-4 by transplanting free islets and exendin-4 treated macroencapsulated rat islets (0.1 nM) prior to transplant or post transplant (100 ng/day, day 0-7). Pre-culture with exendin-4 followed by recipient treatment improved the outcome of both free and macroencapsulated islet grafts. Also exendin-4 enhanced HIF-1α mRNA and protein expression levels. The improved outcome was related to islet graft resistance to hypoxia during the peri-transplant period by increasing the expression of HIF-1α [25].
The Osei group investigated GLP-1 on islet allograft function in diabetic cynomolgus monkeys induced by pancreatectomy, with or without immunosuppression. When exendin-4 was administered pre transplant, the average blood glucose levels at day 5 were significantly lower than those treated following transplant (52.7 ± 14.8 mg/dl vs. 154.3 ± 105.5 mg/dl) and were comparable to the group treated with immunosuppression. IVGTTs showed normal glucose clearance and insulin curves only in the pre-treated exendin-4 and immunosuppression groups [26].
2.2 Incretins promote beta-cells maturation and proliferation
Rabinovitch and colleagues transplanted a low purity endocrine tissue (7 % beta-cells) with an abundance of exocrine (29% duct-cells and 25% acinar cells) into NOD-scid mice (non-obese diabetic-severe combined immune deficiency) and were then treated with GLP-1 and gastrin, which is also capable of stimulating beta-cell growth, for 5 weeks. Mice glucose levels were significantly reduced and insulin and C-peptide levels were increased. Additionally, there was a 4-fold increase in insulin-positive cells in the grafts and the majority of these positive cells were cytokeratin 19-positive, indicating the expanded beta-cell mass mainly from the duct-cells [27]. They also showed a similar result in NOD (non-obese diabetic) mice. Additionally, insulin autoantibodies were reduced. Syngeneic islet grafts exposed to this combinational treatment were shown infiltrated by leukocytes, with a shift in cytokine expression from interferon-gamma to the transforming growth factor-beta1 [28].
The Leung group showed that when mice fetal islet-like cell clusters were transplanted, long-term exendin-4 treatment could improve their maturation and proliferation. Immunoassaying indicated increased lectins and insulin levels [29]. Since immature fetal beta-cells are un-responsive to glucose and their maturation occurs following post-birth oral feeding associated with gut hormones such as GLP-1 and cholecystokinin (CCK), the Tuch group treated pig fetal beta-cells with a combination of GLP-1 (100 nM) and CCK (5 μM) showing that the cells had enhanced glucose-responsive insulin secretion and 1.9-fold increase of PDX-1 positive cells, an indication of differentiation to a beta-cell-like fate [30]. Pig tissues are considered as a potential donor source due to the tissue’s availability and its similar insulin amino acid sequence to human insulin. However there are two major challenges: pig islets have inferior insulin secretion and are less differentiated. The Calafiore group investigated in vitro maturation and differentiation of neonatal pig islets using a combinational high glucose and GLP-1 based on assumptions: both can activate PI3K and protein kinase C that would stimulate cell proliferation, insulin gene expression, and PDX-1 translocation. They showed that neonatal pig pancreatic islets (NPI) treated with 50 nM GLP-1 + 25 mM glucose displayed significant increased expression of PDX1, NKx6.1, pre-proinsulin, and GK [31].
The Hayek group investigated the role of exendin-4 on growth and differentiation of undifferentiated precursors in human islet like cell clusters (ICCs) and showed that exendin-4 up-regulated PDX1 when transplanted into nude mice. The ICCs grafts also had significant higher insulin secretion responses to glucose with detectable human C-peptide [32].
Whether the effects of GLP-1 on beta-cell replication in rodent models can be translated in humans remains largely unknown. The Guo group compared exendin-4 on beta-cell replication in mouse and human islet by transplanting both mouse and human islets from varying ages into diabetic mice and then given bromodeoxyuridine (BrdU) for 4 weeks. Although diabetes was reversed in all mice transplanted using syngeneic mouse islets from both young or old donors, normoglycemia was achieved significantly faster under exendin-4 (10 ± 7 days) vs. control (16 ± 4 days) and the grafts had significantly higher insulin+/BrdU+beta-cells. Human islet from ≤ 22-year-old donors had a similar result (16.2 ± 5.2%) compared to the control (7.2 ± 1.8%), but not from donors ≥ 35-year-old [33].
Oleoylethanolamide (OEA, a GLP-1R endogenous ligand) and PSN632408 (a GRL-1R synthetic agonist) have been investigated for promoting beta-cell replication. One study showed that both significantly increased a dose-dependent increase of insulin+/BrdU+ beta-cells in vitro. All diabetic recipient mice, given marginal syngeneic islet transplants with OEA or PSN632408, achieved significantly faster normoglycemia and a higher percentage of insulin+/BrdU+ beta -cells in islet grafts [34].
2.3 Incretins modulate immunoregulation, inflammatory reaction, and apoptosis
It has been shown that common immunosuppressants impair beta-cell function [35-37]. The Perfetti group reported that mouse insulinoma (MIN6) cells overexpressed with GIP-1 were more resistant to the toxicity caused by an immunosuppression cocktail of tacrolimus, rapamycin, and mycophenolate. Additionally, GLP-1 increased the expression of the antiapoptotic protein (BCL-2) and decreased the abundance of proapoptotic markers (PARP-p85 and Smac/Diablo) [38]. The Perfettit group also showed that transfection of MIN-6 cells with a minigene encoding for the human GLP-1 under the control of a rat insulin promoter, followed by alginate microencapsulation, resulted in excellent survival and function when transplanted, even without immunosuppression [39]. Calcineurin, a calcium-regulated phosphatase, can intersect with both the calcium and cAMP-mediated signaling pathways. The Soleimanpour group reported that human beta-cell apoptosis was significantly increased and rodent beta-cell replication was decreased under tacrolimus. However, exendin-4 nearly reversed the human beta-cell survival and rodent beta-cell replication caused by tacrolimus. Additionally, these cytoprotective effects were linked to the restoration of the insulin receptor substrate-2 (Irs2) expression inhibited by tacrolimus. Irs2 is a known cAMP-responsive element-binding protein target and upstream regulator of the PI3K/Akt pathway [40].
The Pastori group investigated the role of GLP-1’s anti-inflammatory properties in human islets and showed that exendin-4 significantly reduced the content of inflammation-related molecules (tissue factor, IFN- γ, IL-17, IL-1β, and IL-2) and caspase 3 activation, whereas it also increased phosphorylation of ERK1/2, STAT3, and Akt in vitro. Additionally, exendin-4 could induce serine proteinase inhibitor 9 (PI-9) levels in human islets in vitro and also in vivo islet grafts when transplanted into immunodeficient mice. Interestedly, the PI-9 induction could be partially blocked in vitro by antagonist Exendin-9, a GLP-1R antagonist [41].
Although beyond the scope of this review, it would be worth noting that GLP-1 and derivates, often used as adjunctive therapy synergistically with other immunoregulators, have been studied extensively in Type I diabetes animal models such as nonobese diabetic (NOD) mice for understanding GLP-1 immunoregulatory and anti-inflammatory properties. These studies have several following important findings: GLP-1 can enhance remission of T1DM in NOD mice treated with anti-CD3 mAb by increasing the recovery of the residual islet mass [42]. GLP-1 synergistically augments diabetic remission-induced by anti-lymphocyte serum (ALS) [43]. Combining Complete Freund’s adjuvant (CFA) with GLP-1 significantly increases the insulin content and BrdU positive cells in NOD islets [44]. DPP-IV inhibitor can reverse new-onset diabetes in NOD mice by reducing insulitis, increasing CD4(+)CD25(+) FoxP3(+) regulatory T cells; and stimulating beta-cell replication [45]. Continuous infusion of GLP-1 to prediabetic NOD mice induces beta-cell proliferation and delays the onset of TIDM [46].
2.4 Incretin overexpression in vivo and in vitro
Clinical efficacy of GLP-1 is greatly hampered by its rapid degradation and clearance, as well as its side effects and injected doses that are tolerable to patients. The dose needed to promote beta-cell proliferation in rodents, is typically between 50-100 μg/kg that is far beyond the dose tolerable to humans (< 2 μg/kg) and greatly exceeds the dose required in TIIDM (5 μg, BID). GLP-1 is produced from the proglucagon precursor by PC1/3. In α-cells, proglucagon is differentially processed by PC2 to release glucagon, leaving GLP-1 trapped within a larger fragment with unknown function. PC1/3 expression can be activated in α-cells under certain conditions such as partial pancreatectomy and induction of diabetes. The Kieffer group showed that the induction of PC1/3, through exogenous adenoviral delivery into α-cells, resulted in increased insulin secretion, improved islet function in response to cytokines, and enhanced graft function by enhancing PDX1 and insulin content. These results demonstrated a unique strategy for liberating GLP-1 from directly within target organ and highlighted the potential for up-regulating GLP-1 for treating diabetes [47]. The group also showed that the transplantation of encapsulated PC1/3-expressing alpha TC-1 cells into diabetic mice increased plasma GLP-1/GLP-2 levels, improved glucose tolerance, and promoted beta-cell proliferation; however, not from PC2-expressing alpha-cells [48]. Transduction of a recombinant adenovirus vector expressing GLP-1 has also been shown to increase bioactive GLP-1, resistance to H2O2, and insulin secretion. Post-transplant analysis revealed that the rAd-GLP-1-transduced islets had more Ki67-positive cells. Diabetic mice transplanted with a marginal mass of rAd-GLP-1-transduced islets became normoglycemic more rapidly and 78% of the recipients were normoglycemic, whereas only 48% in control [49].
2.5 Long half-life GLP-1 analog
The Sharpiro group was first to investigate liraglutide in a transplant model. They showed that mice transplanted with a marginal syngeneic islet mass and treated with liraglutide (200 μ/kg) reversed diabetes in a shorter time (median 1 vs. 7 d; p = 0.0003), even in the recipients receiving sirolimus (median 1 vs. 72.5 d; p < 0.0001). Liraglutide also improved glucose clearance and reduced beta-cell apoptosis. Interestingly, the benefits were diminished after liraglutide discontinued or followed a late-start therapy post transplant [50]. In porcine islets, liraglutide did not have any advantage on the insulin independence rate. However, a significant increase in insulin secretion was observed during glucose challenge [51]. This result was contradicted to their previous findings in rodents that may be due to species difference.
The culturing of human islets is often associated with significant cell loss that may prevent islet transplantation from taking place. The Shapiro group showed when cultured islets with liraglutide (1 μM), islet mass was preserved, especially larger islets and was related to apoptosis prevention. Furthermore, at a dose of 200 μg/kg the liraglutide-treated islets had improved islet graft function in C57Bl/6-RAG−/− mice [52].
2.6 DPP-IV inhibitor for prolonging incretin circulation levels
DPP-IV inhibitors were introduced for diabetes treatment due to their ability to block GLP-1 degradation, and they can promote beta-cell proliferation and survival. The Mclntosh group studied the effects of the MK0431 (sitagliptin) in a diabetic transplant mouse model and demonstrated that MK0431 could fully regulate blood glucose levels, accompanied with a reduction in DPP-IV levels. PET imaging demonstrated a profound protective effect by MK0431 on islet graft size [53]. Again, they showed that MK0431-treated islets prior to transplant prolonged graft survival in NOD mice, whereas the treatment after transplantation resulted in only a small beneficial effect. Furthermore, MK0431 could prevent insulitis and reduced in vitro migration of isolated splenic CD4+ T-cells and prevent autoimmunity on graft survival partially by decreasing homing of CD4+ T-cells into beta-cells involving cAMP/PKA/Rac1 activation [54]. In 70% pancreatectomized diabetic mice, sitagliptin also increased active GLP-1 and improved glycemic control, especially under islet transplantation. Additionally, the beta-cell mass of the pancreatic remnants increased [55]
2.7 Application of GLP-1 derivatives for pancreatic beta-cell imaging
Since the structural and biochemical interaction between GLP-1 and its receptor have been well characterized, GLP-1 is considering a suitable peptide which can be used to develop as peptide-based and non-invasive imaging probes for detecting and monitoring pancreatic beta-cell mass in vivo. GlP-1R is highly expressed on beta-cell surface and the receptor has high binding affinity at nanomolar concentration. One limiting factor for such application is that GLP-1 has short biological half-life time (1-2 min) due to rapid enzymatic degradation. In order to develop GLP-1-based diagnostics, extensive studies have been conducted to increase the biological half-life of the parent peptide through chemical modifications resulting promising findings in many research and clinical areas including some for in vivo imaging and monitoring islet grafts [56-59]. Since the application of GIP-1 derivates as imaging tool is not central focus of this review and has been previously reviewed, we will not discuss these findings in details.
3. Human clinical trials and epidemiologic studies
GLP-1 is a suitable candidate for an adjuvant therapy for islet transplant as it can induce insulin secretion, preserve islet graft mass, and have anti-apoptotic effects. Exendin-4 has been extensively studied for TIIDM treatment, but only a handful of studies have been reported for islet transplant. Liraglutide and DPP-IV inhibitors are, so far, only used for TIIDM, however there are several ongoing clinical trials that have yet to report their results.
In 2006, the Meneilly group evaluated the effects of GLP-1 on the insulin secretion in islet transplanted TIDM patients using a hyperglycemic glucose clamp protocol and compared to both normal control and TIIDM patients [60]. They showed that GLP-1 had a significant incretin effect on the transplanted islets; however the response was less than the controls with stimulated insulin levels of 2108 pM ± 344 in normal control, 929 pM ± 331 in TIIDM, and 329 pM ± 112 in islet transplants. Although first phase insulin release was absent, second phase insulin was not significantly reduced (99 pM ± 18) when compared to other groups (118 pM ± 29 in control and 68 pM ± 20 in TIIDM).
In 2007, the Thompson group analyzed GLP-1 efficacy for glucose homeostasis on eleven C-peptide positive transplant recipients with elevated glucose levels [61]. Two patients achieved good glycemic control and insulin independence. One patient who had received 5500 IEq/kg in the first islet infusion was able to stop using insulin. Seven other patients decreased their insulin dose by 39%. Hyperglycemic clamp studies showed a rise in the second phase of insulin release (before exenatide: 246 pM ± 88; during exenatide: 644 pM ± 294). Meal tolerance studies performed either during or post exendin-4 treatment did not indicate a difference in glucose and C-peptide values. More interestingly, no trophic effect on islet grafts was observed. It is worth noting that common side effects were observed in all patients including nausea and vomiting.
In 2008, the Alejandro group analyzed sixteen islet transplant subjects under exendin-4. Twenty five percent of the patients discontinued due to side effects while 12 finished (follow-up for 214 ± 57 days). When compared to prior treatment, their insulin requirements at six months were significantly reduced (0.15 ± 0.02 vs. 0.11 ± 0.025 U/kg per day; p < 0.0001) with stable glycemic control. Glycemia was also significantly decreased and C-peptide-to-glucose ratio was increased significantly at 5th and 6th months (ratio, 1.09 ± 0.15 vs. 1.52 ± 0.18; p < 0.05). Improved glucose disposal (glucose AUC: 52,332 mg/min/dL ± 3,219 vs. 42,072 mg/min/dL ± 1,965; p = 0.002) and C-peptide secretion were observed. It also showed an increase in the mixed meal stimulation index (0.50 ± 0.06 vs. 0.66 ± 0.09 pmol/mL; p = 0.03), as well as a marked suppression of glucagon secretion and a progressive increase in amylin secretion. The side effects were more frequent and severe compared to other reports in TIIDM patients and the tolerated doses were also much lower than previous data.
In a single-arm and nonrandomized study, the Alejandro group analyzed exendin-4 effects on long-term metabolic and hormonal regulation on eleven islet transplant recipients who lost graft function [62]. They showed that exendin-4 improved glucose homeostasis, increased the amylin/insulin ratio, and decreased proinsulin/insulin ratio. Exendin-4 administration 1 hr before MMTT showed decreased levels of glucagon and glucose at 0 min and attenuated in their postprandial rise. Time-to-peak glucose was delayed, followed by insulin, proinsulin, amylin, and C-peptide, indicating glucose-driven insulin secretion. However, the glucose and glucagon suppression responses during MMTT with exendin-4 were no longer observed after 12-month follow-up. Interestingly, different degrees of responsiveness to exendin-4 were observed among the patients. The important finding based on this study indicated that exendin-4 was highly beneficial in sustaining long-term islet graft survival and further benefits could be gained from exendin-4 application at all stages of islet preparation, transplantation, and post transplant follow-up.
The Naji group also studied GLP-1 effects on islet transplant recipients (n = 5) and whole pancreas transplant recipients (n = 6) [63]. All subjects were evaluated by glucose-potentiated arginine testing (5 g arginine injected under the basal and hyperglycemic clamp conditions) with either GLP-1 (1.5 pmol/kg/min) or placebo. Under exendin-4, basal glucose was lower and accompanied with increased insulin and decreased glucagon in both the groups. During the hyperglycemic clamp, a significantly greater glucose infusion rate was required with GLP-1 vs. placebo in both groups; however this was more pronounced in the pancreas than in the islet group. The increased glucose infusion rate was associated with significant increases in second-phase insulin secretion that also tended to be much greater in the pancreas than in the islet group, whereas glucagon was equivalently suppressed by the hyperglycemic clamp in the GLP-1 and placebo groups. The GLP-1-induced increase in second-phase insulin correlated well with the beta-cell secretory capacity. Additionally, the proinsulin secretory ratio (PISR) during the glucose-potentiated arginine test was significantly greater in both GLP-1 groups. However, the degree of GLP-1 effects on insulin secretion depends heavily on the functional beta-cell mass.
In our study, ten islet transplant recipients were divided into two groups: group I under the Edmonton immunosuppression protocol consisting of daclizumab, sirolimus, and tacrolimus (n = 4; total IEq = 1,460,080 ± 418,330/patient) and group II used the UIC protocol consisting of the Edmonton protocol + etanercept + exendin-4 (n = 6; Total IEq = 537,495 ± 190,968/patient). Like the group I patients, the group II patients also had reversed diabetes, but with a much smaller total islet mass required. Both the HbA1c and the HYPO score were similar. In agreement with other studies, no evidence of beta-cell proliferation was observed based on the results of the OGTT and IVGTT challenge and proinsulin to insulin ratio. This study indicated that the utilization of exendin-4 in conjunction with islet transplant showed promise in decreasing the need for multiple donors.
Health related quality of life (HRQoL) is one of the most important outcomes to measure effectiveness of an intervention. The Alejandro group studied retrospectively 40 islet transplant recipients who completed 344 Health Status Questionnaires (HSQ2.0) and 384 Diabetes quality of life questionnaires (DQoL), showing that exendin-4 has positive effects on mental health and health perception scales of HSQ 2.0 in all patients regardless of islet infusion number, islet alone vs. islet after kidney transplantation, those who have longer diabetes duration, or higher insulin dosage. Furthermore, a lower rate of severe adverse events has been found in this study [64].
Conclusion
Incretins are promising new adjunct drugs for islet transplantation, based both on their working mechanism (Fig.1) and currently available clinical data. Although there are no recommended guidelines, both laboratory and clinical data suggest that incretin should be administered as early as possible, probably during islet culture or peri-islet transplant, to achieve optimal outcomes. Current data remains unclear whether these benefits are durable and long-term. Beyond glycemic control with minimal hypoglycemic risk, incretin-based therapy has various beta-cell cytoprotective effects, but this is confirmed or defined more in vitro and animal model than in human subjects. In allogenic islet transplant, there are two unavoidable factors: immunotoxicity and hypoxia. Recently, it has been shown that GLP-1 stimulates insulin biosynthesis, beta-cell growth, and proliferation via GLP-1-cAMP-mTOR-HIF pathways [25, 65-68]. Sirolimus is a potent inhibitor for mTOR and a key component in the Edmonton protocol. Together, it may explain in part the reason why GLP-1 only has acute insulin secretion effects in the transplanted grafts while long-term or trophic effects were seldom observed in human studies. Another probable reason is that the doses used in vivo and animal studies are much higher than that human are tolerate. Again, clinical data of incretins long-term safety and tolerability levels is largely lacking. Closer emphasis should be placed on this data since the GLP-1R is expressed on a broad range of tissue types and therefore a wide range of different (side) effects may potentially occur. There are several studies linking exendin-4 to pancreatitis [69-71], pancreatic and thyroid cancer [71-73] while no definite relationship has been determined [74, 75]. A recent publication on the human pancreatic tissue from the TIIDM patients treated with incretin showed again that incretin therapy led marked expansion of exocrine and endocrine pancreas accompanied with exocrine dysplasia and potential for glucagon-producing neuroendocrine tumors [76]. For this reason, large-sized longitudinal studies and close clinical monitoring in islet transplanted patients are warranted. Of equal importance, we have to understand in vivo dosage tolerability and develop a formulation with a much longer half-life to increase tolerability that can be achieved by designing a new incretin class with a higher efficacy and fewer side effects. Last, but not least, special care of transplanted islets subjected to the drugs is needed, especially as they are high-risk individuals who are often on various immunosuppressant medications and have complications from diabetes.
Figure 1.
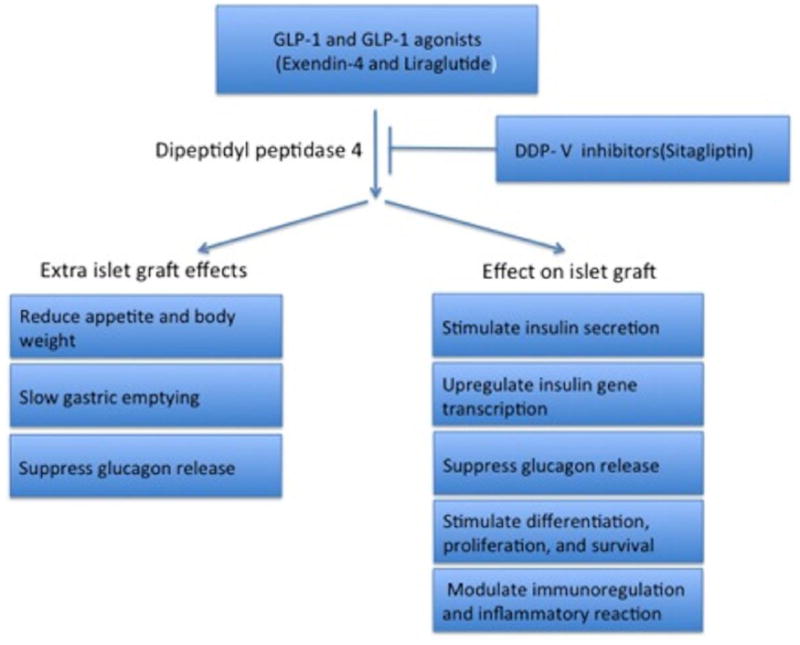
Working mechanisms of GLP-1, GLP-1 agonist, and DPPV-4 inhibitor for islet transplantation.
Acknowledgments
This work was supported in part by the Chicago Diabetes Project (CDP) and a start up grant by University of Illinois at Chicago College Medicine (JO).
Footnotes
Conflict of Interest
Yong Wang, Meirigeng Qi, James J. McGarrigle, Brian Rady, Maureen Davis, Pilar Vaca, and Jose Oberholzer declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Yong Wang, wangy@uic.edu. 312-996-0851(W), 312-996-7913(Fax). Department of Surgery/Transplant, University of Illinois at Chicago, Chicago, IL 60612.
Meirigeng Qi, mrgqi@uic.edu. 312-996-0530(W), 312-996-7913(Fax). Department of Surgery/Transplant, University of Illinois at Chicago, Chicago, IL 60612.
James J. McGarrigle, James_macg@hotmail.com. 312-996-8316(W), 312-996-7913(Fax). Department of Surgery/Transplant, University of Illinois at Chicago, Chicago, IL 60612.
Brian Rady, brady@uic.edu 312-996-8316(W), 312-996-7913(Fax). Department of Surgery/Transplant, University of Illinois at Chicago, Chicago, IL 60612.
Maureen Davis, medavis11@gmail.com. 312-996-8316(W), 312-996-7913(Fax). Department of Surgery/Transplant, University of Illinois at Chicago, Chicago, IL 60612.
Pilar Vaca, Vaca.pilar@gmail.com. 312-996-8316(W), 312-996-7913(Fax). Department of Surgery/Transplant, University of Illinois at Chicago, Chicago, IL 60612.
Jose Oberholzer, jober@uic.edu. 312-996-6771(W), 312-996-7961(Fax). Department of Surgery/Transplant, University of Illinois at Chicago, Chicago, IL 60612.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Lakey JR, Paty BW, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51(7):2148–2157. doi: 10.2337/diabetes.51.7.2148. [DOI] [PubMed] [Google Scholar]
- 3.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35(7):1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangemi A, Salehi P, Hatipoglu B, et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8(6):1250–1261. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- 5.Alejandro R, Barton FB, Hering BJ, et al. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86(12):1783–1788. doi: 10.1097/TP.0b013e3181913f6a. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 7.Ryan EA, Paty BW, Senior PA, et al. Beta-score: an assessment of beta-cell function after islet transplantation. Diabetes Care. 2005;28(2):343–347. doi: 10.2337/diacare.28.2.343. [DOI] [PubMed] [Google Scholar]
- 8.Danielson KK, Hatipoglu B, Kinzer K, et al. Reduction in Carotid Intima-Media Thickness After Pancreatic Islet Transplantation in Patients With Type 1 Diabetes. Diabetes Care. 2012 doi: 10.2337/dc12-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibly RF, Graham JG, Luo X, et al. Advancing islet transplantation: from engraftment to the immune response. Diabetologia. 2011;54(10):2494–2505. doi: 10.1007/s00125-011-2243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro AM, Lakey JR, Paty BW, et al. Strategic opportunities in clinical islet transplantation. Transplantation. 2005;79(10):1304–1307. doi: 10.1097/01.tp.0000157300.53976.2a. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Moore DJ, Ketchum RJ, et al. Resolving the conundrum of islet transplantation by linking metabolic dysregulation, inflammation, and immune regulation. Endocr Rev. 2008;29(5):603–630. doi: 10.1210/er.2008-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 13.Tanizawa Y, Kaku K. Human glucagon-like peptide-1 receptor gene in NIDDM. Nihon Rinsho. 1994;52(10):2731–2736. [PubMed] [Google Scholar]
- 14.Holz CGt, Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37) Nature. 1993;361(6410):362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skoglund G, Hussain MA, Holz GG. Glucagon-like peptide 1 stimulates insulin gene promoter activity by protein kinase A-independent activation of the rat insulin I gene cAMP response element. Diabetes. 2000;49(7):1156–1164. doi: 10.2337/diabetes.49.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baggio L, Kieffer TJ, Drucker DJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, regulates fasting glycemia and nonenteral glucose clearance in mice. Endocrinology. 2000;141(10):3703–3709. doi: 10.1210/endo.141.10.7720. [DOI] [PubMed] [Google Scholar]
- 17.Xu G, Stoffers DA, Habener JF, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 18.Stoffers DA, Kieffer TJ, Hussain MA, et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49(5):741–748. doi: 10.2337/diabetes.49.5.741. [DOI] [PubMed] [Google Scholar]
- 19.van der Burg MP, van Suylichem PT, Guicherit OR, et al. Glucoregulation after canine islet transplantation: contribution of insulin secretory capacity, insulin action, and the entero-insular axis. Cell Transplant. 1997;6(5):497–503. doi: 10.1177/096368979700600509. [DOI] [PubMed] [Google Scholar]
- 20.van der Burg MP, van Suylichem PT, Guicherit OR, et al. The relative contribution of insulin secretory capacity, insulin action, and incretins to metabolic control after islet transplantation in dogs. J Mol Med (Berl) 1999;77(1):104–106. doi: 10.1007/s001090050312. [DOI] [PubMed] [Google Scholar]
- 21.King A, Lock J, Xu G, et al. Islet transplantation outcomes in mice are better with fresh islets and exendin-4 treatment. Diabetologia. 2005;48(10):2074–2079. doi: 10.1007/s00125-005-1922-0. [DOI] [PubMed] [Google Scholar]
- 22•.Toyoda K, Okitsu T, Yamane S, et al. GLP-1 receptor signaling protects pancreatic beta cells in intraportal islet transplant by inhibiting apoptosis. Biochem Biophys Res Commun. 2008;367(4):793–798. doi: 10.1016/j.bbrc.2008.01.046. The study reports a beneficial effect of GLP-1 in restoration of normoglycemia in a minimal transplant rodent model and also indicated the benefits were achieved via reduction the number of apoptotic beta-cells during early post-transplant phas. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A, Sorenby A, Wernerson A, et al. Exendin-4 treatment improves metabolic control after rat islet transplantation to athymic mice with streptozotocin-induced diabetes. Diabetologia. 2006;49(6):1247–1253. doi: 10.1007/s00125-006-0251-2. [DOI] [PubMed] [Google Scholar]
- 24•.Juang JH, Kuo CH, Wu CH, et al. Exendin-4 treatment expands graft beta-cell mass in diabetic mice transplanted with a marginal number of fresh islets. Cell Transplant. 2008;17(6):641–647. doi: 10.3727/096368908786092766. This study reports in vivo islet graft function improvement by exendin-4 in a marginal number rodent transplant model with higher cure rate and shorter time to reach normoglycemia. [DOI] [PubMed] [Google Scholar]
- 25••.Jia X, Sharma A, Kumagai-Braesch M, et al. Exendin-4 increases the expression of hypoxia-inducible factor-1alpha in rat islets and preserves the endocrine cell volume of both free and macroencapsulated islet grafts. Cell Transplant. 2012;21(6):1269–1283. doi: 10.3727/096368911X627408. The study links the beneficial effect of GLP-1 with increased levels of HIF-1a mRNA and protein expression, indicating that GLP-1 improves islet graft resistance to hypoxia peri-transplant period by increasing HIF-1a. [DOI] [PubMed] [Google Scholar]
- 26••.Buss JL, Rajab A, Essig ED, et al. Exenatide pretreatment improved graft function in nonhuman primate islet recipients compared to treatment after transplant only. J Transplant. 2012;2012:382518. doi: 10.1155/2012/382518. This study reports in monkey transplant model that exendin-4 treatment prior to transplant has more beneficial effects on islet graft function than post-transplant treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Suarez-Pinzon WL, Lakey JR, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin induces beta-cell neogenesis from pancreatic duct cells in human islets transplanted in immunodeficient diabetic mice. Cell Transplant. 2008;17(6):631–640. doi: 10.3727/096368908786092775. This study shows that combination therapy with GLP-1 and gastrin expands the beta-cell mass in human islets implanted in NOD mice, largely from pancreatic duct cells associated within the islets and improves islet graft function. [DOI] [PubMed] [Google Scholar]
- 28••.Suarez-Pinzon WL, Power RF, Yan Y, et al. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes. 2008;57(12):3281–3288. doi: 10.2337/db08-0688. This report shows that combination therapy with GLP-1 and gastrin restores normoglycemia in diabetic NOD mice by increasing insulin-positive cells, reducing beta-cell apoptosis, and down-regulating the autoimmune response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suen PM, Li K, Chan JC, et al. In vivo treatment with glucagon-like peptide 1 promotes the graft function of fetal islet-like cell clusters in transplanted mice. Int J Biochem Cell Biol. 2006;38(5-6):951–960. doi: 10.1016/j.biocel.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Hardikar AA, Wang XY, Williams LJ, et al. Functional maturation of fetal porcine beta-cells by glucagon-like peptide 1 and cholecystokinin. Endocrinology. 2002;143(9):3505–3514. doi: 10.1210/en.2001-211344. [DOI] [PubMed] [Google Scholar]
- 31.Mancuso F, Basta G, Calvitti M, et al. Long-term cultured neonatal porcine islet cell monolayers: a potential tissue source for transplant in diabetes. Xenotransplantation. 2006;13(4):289–298. doi: 10.1111/j.1399-3089.2006.00305.x. [DOI] [PubMed] [Google Scholar]
- 32.Movassat J, Beattie GM, Lopez AD, et al. Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J Clin Endocrinol Metab. 2002;87(10):4775–4781. doi: 10.1210/jc.2002-020137. [DOI] [PubMed] [Google Scholar]
- 33••.Tian L, Gao J, Weng G, et al. Comparison of exendin-4 on beta-cell replication in mouse and human islet grafts. Transpl Int. 2011;24(8):856–864. doi: 10.1111/j.1432-2277.2011.01275.x. This study shows that under exendin-4 treatment, mouse islet grafts have more beta-cell proliferation than human islet grafts. In human islet grafts, extent of beta-cell proliferation is dependent on donor age. [DOI] [PubMed] [Google Scholar]
- 34.Gao J, Tian L, Weng G, et al. Stimulating beta-cell replication and improving islet graft function by AR231453, A gpr119 agonist. Transplant Proc. 2011;43(9):3217–3220. doi: 10.1016/j.transproceed.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Barlow AD, Xie J, Moore CE, et al. Rapamycin toxicity in MIN6 cells and rat and human islets is mediated by the inhibition of mTOR complex 2 (mTORC2) Diabetologia. 2012;55(5):1355–1365. doi: 10.1007/s00125-012-2475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanemura M, Ohmura Y, Deguchi T, et al. Rapamycin causes upregulation of autophagy and impairs islets function both in vitro and in vivo. Am J Transplant. 2012;12(1):102–114. doi: 10.1111/j.1600-6143.2011.03771.x. [DOI] [PubMed] [Google Scholar]
- 37.Johnson JD, Ao Z, Ao P, et al. Different effects of FK506, rapamycin, and mycophenolate mofetil on glucose-stimulated insulin release and apoptosis in human islets. Cell Transplant. 2009;18(8):833–845. doi: 10.3727/096368909X471198. [DOI] [PubMed] [Google Scholar]
- 38.D’Amico E, Hui H, Khoury N, et al. Pancreatic beta-cells expressing GLP-1 are resistant to the toxic effects of immunosuppressive drugs. J Mol Endocrinol. 2005;34(2):377–390. doi: 10.1677/jme.1.01655. [DOI] [PubMed] [Google Scholar]
- 39.Aoki T, Hui H, Umehara Y, et al. Intrasplenic transplantation of encapsulated genetically engineered mouse insulinoma cells reverses streptozotocin-induced diabetes in rats. Cell Transplant. 2005;14(6):411–421. doi: 10.3727/000000005783982990. [DOI] [PubMed] [Google Scholar]
- 40.Soleimanpour SA, Crutchlow MF, Ferrari AM, et al. Calcineurin signaling regulates human islet {beta}-cell survival. J Biol Chem. 2010;285(51):40050–40059. doi: 10.1074/jbc.M110.154955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Cechin SR, Perez-Alvarez I, Fenjves E, et al. Anti-Inflammatory Properties of Exenatide in Human Pancreatic Islets. Cell Transplant. 2011 doi: 10.3727/096368911X576027. [Epub ahead of print]. This report describes anti-inflammatory and cytoprotective properties of exendin-4 in human islets by reducing tissue factor, IFN-γ, IL-17, IL-1β, IL-2, caspase 3 activation, whereas increased phosphorylation of ERK1/2, STAT3 and Akt. Also induction of serine proteinase inhibitor 9 PI-9 was detecte. [DOI] [PubMed] [Google Scholar]
- 42.Sherry NA, Chen W, Kushner JA, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology. 2007;148(11):5136–5144. doi: 10.1210/en.2007-0358. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa N, List JF, Habener JF, et al. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes. 2004;53(7):1700–1705. doi: 10.2337/diabetes.53.7.1700. [DOI] [PubMed] [Google Scholar]
- 44.Tian B, Hao J, Zhang Y, et al. Upregulating CD4+CD25+FOXP3+ regulatory T cells in pancreatic lymph nodes in diabetic NOD mice by adjuvant immunotherapy. Transplantation. 2009;87(2):198–206. doi: 10.1097/TP.0b013e3181933261. [DOI] [PubMed] [Google Scholar]
- 45.Tian L, Gao J, Hao J, et al. Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology. 2010;151(7):3049–3060. doi: 10.1210/en.2010-0068. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Tokui Y, Yamagata K, et al. Continuous stimulation of human glucagon-like peptide-1 (7-36) amide in a mouse model (NOD) delays onset of autoimmune type 1 diabetes. Diabetologia. 2007;50(9):1900–1909. doi: 10.1007/s00125-007-0737-6. [DOI] [PubMed] [Google Scholar]
- 47.Wideman RD, Yu IL, Webber TD, et al. Improving function and survival of pancreatic islets by endogenous production of glucagon-like peptide 1 (GLP-1) Proc Natl Acad Sci U S A. 2006;103(36):3468–113473. doi: 10.1073/pnas.0600655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wideman RD, Covey SD, Webb GC, et al. A switch from prohormone convertase (PC)-2 to PC1/3 expression in transplanted alpha-cells is accompanied by differential processing of proglucagon and improved glucose homeostasis in mice. Diabetes. 2007;56(11):2744–2752. doi: 10.2337/db07-0563. [DOI] [PubMed] [Google Scholar]
- 49•.Chae HY, Kang JG, Kim CS, et al. Effect of glucagon-like peptide-1 gene expression on graft function in mouse islet transplantation. Transpl Int. 2012;25(2):242–249. doi: 10.1111/j.1432-2277.2011.01394.x. The study shows that delivery of the GLP-1 gene using adenovirus to islets enhances islet cell survival during the early post-transplant period and preserves islet mass and graft functions with marginal mass transplant model. [DOI] [PubMed] [Google Scholar]
- 50••.Merani S, Truong W, Emamaullee JA, et al. Liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose homeostasis in marginal mass islet transplantation in mice. Endocrinology. 2008;149(9):4322–4328. doi: 10.1210/en.2008-0501. This report demonstrates that liraglutide, a long-acting human glucagon-like peptide 1 analog, improves glucose-dependent insulin secretion and has a beneficial impact on the engraftment and function of syngeneic islet transplants in mice, especially in early administration. [DOI] [PubMed] [Google Scholar]
- 51•.Emamaullee JA, Merani S, Toso C, et al. Porcine marginal mass islet autografts resist metabolic failure over time and are enhanced by early treatment with liraglutide. Endocrinology. 2009;150(5):2145–2152. doi: 10.1210/en.2008-1116. This study shows that incubation with liraglutide enhanced porcine islet survival and function after prolonged culture. Although no improvement of insulin independence rate, liraglutide significant increases in insulin secretion and acute-phase insulin response. [DOI] [PubMed] [Google Scholar]
- 52•.Toso C, McCall M, Emamaullee J, et al. Liraglutide, a long-acting human glucagon-like peptide 1 analogue, improves human islet survival in culture. Transpl Int. 2010;23(3):259–265. doi: 10.1111/j.1432-2277.2009.00984.x. The report demonstrates that human islets cultured with liraglutide increases islet mass, especially for large islets and reduces beta-cell apoptosis. In vivo continuous administration of liraglutide warrants long-term graft function. [DOI] [PubMed] [Google Scholar]
- 53••.Kim SJ, Nian C, Doudet DJ, et al. Inhibition of dipeptidyl peptidase IV with sitagliptin (MK0431) prolongs islet graft survival in streptozotocin-induced diabetic mice. Diabetes. 2008;57(5):1331–1339. doi: 10.2337/db07-1639. This study shows that sitagliptin, a DPP-IV inhibitor, prolongs islet graft survival in an animal model of Type I diabetes associated with a profound protective effect on islet sizes. [DOI] [PubMed] [Google Scholar]
- 54••.Kim SJ, Nian C, Doudet DJ, et al. Dipeptidyl peptidase IV inhibition with MK0431 improves islet graft survival in diabetic NOD mice partially via T-cell modulation. Diabetes. 2009;58(3):641–651. doi: 10.2337/db08-1101. This study shows that sitagliptin treatment results a prolonged graft function of the transplanted islets in diabetic NOD mice, especially applied prior to early administration and sitagliptin reduces insulitis in NOD mice by decreasing the homing of CD4+ T-cells into pancreatic beta-cells involving cAMP/PKA/RAC1 activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Kim YS, Oh SH, Park KS, et al. Improved outcome of islet transplantation in partially pancreatectomized diabetic mice by inhibition of dipeptidyl peptidase-4 with sitagliptin. Pancreas. 2011;40(6):855–860. doi: 10.1097/MPA.0b013e318214832d. This report shows that DPP-4 inhibitor favorably affects islet transplant outcomes in partially pancreatectomized diabetic mice by increasing beta-cell proliferation and inhibition beta-cell apoptosis. [DOI] [PubMed] [Google Scholar]
- 56.Reiner T, Thurber G, Gaglia J, et al. Accurate measurement of pancreatic islet beta-cell mass using a second-generation fluorescent exendin-4 analog. Proc Natl Acad Sci U S A. 2011;108(31):12815–12820. doi: 10.1073/pnas.1109859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Z, Todorov I, Li L, et al. In vivo imaging of transplanted islets with 64Cu-DO3A-VS-Cys40-Exendin-4 by targeting GLP-1 receptor. Bioconjug Chem. 2011;22(8):1587–1594. doi: 10.1021/bc200132t. [DOI] [PubMed] [Google Scholar]
- 58.Pattou F, Kerr-Conte J, Wild D. GLP-1-receptor scanning for imaging of human beta cells transplanted in muscle. N Engl J Med. 2010;363(13):1289–1290. doi: 10.1056/NEJMc1004547. [DOI] [PubMed] [Google Scholar]
- 59.Connolly BM, Vanko A, McQuade P, et al. Ex vivo imaging of pancreatic beta cells using a radiolabeled GLP-1 receptor agonist. Mol Imaging Biol. 2012;14(1):79–87. doi: 10.1007/s11307-011-0481-7. [DOI] [PubMed] [Google Scholar]
- 60.Fung M, Thompson D, Shapiro RJ, et al. Effect of glucagon-like peptide-1 (7-37) on beta-cell function after islet transplantation in type 1 diabetes. Diabetes Res Clin Pract. 2006;74(2):189–193. doi: 10.1016/j.diabres.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 61.Ghofaili KA, Fung M, Ao Z, et al. Effect of exenatide on beta cell function after islet transplantation in type 1 diabetes. Transplantation. 2007;83(1):24–28. doi: 10.1097/01.tp.0000251379.46596.2d. [DOI] [PubMed] [Google Scholar]
- 62••.Faradji RN, Tharavanij T, Messinger S, et al. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;86(12):1658–1665. doi: 10.1097/TP.0b013e31818fe448. This clinical study shows that application of exendin-4 in islet transplant patients who lost graft function and insulin independence helps the restoration of islet graft function. Also exendin-4 improves first and second phase insulin release in response to glucose challenge and suppresses postprandial hyperglycemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Rickels MR, Mueller R, Markmann JF, et al. Effect of glucagon-like peptide-1 on beta- and alpha-cell function in isolated islet and whole pancreas transplant recipients. J Clin Endocrinol Metab. 2009;94(1):181–189. doi: 10.1210/jc.2008-1806. This clinical study shows that in both islet and pancreas transplant patients, GLP-1 induces enhancement of glucose-dependent insulin secretion, but not glucagon suppression and the effect is depend on the functional beta-cell mass. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Tharavanij T, Betancourt A, Messinger S, et al. Improved long-term health-related quality of life after islet transplantation. Transplantation. 2008;86(9):1161–1167. doi: 10.1097/TP.0b013e31818a7f45. This retrospective clinical survey indicates that exendin-4 has positive effects on mental health and health perception scales of HSQ 2.0 of islet transplant patients regardless of islet infusion number, islet alone or islet after kidney transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwon G, Marshall CA, Pappan KL, et al. Signaling elements involved in the metabolic regulation of mTOR by nutrients, incretins, and growth factors in islets. Diabetes. 2004;53(Suppl 3):S225–232. doi: 10.2337/diabetes.53.suppl_3.s225. [DOI] [PubMed] [Google Scholar]
- 66••.Van de Velde S, Hogan MF, Montminy M. mTOR links incretin signaling to HIF induction in pancreatic beta cells. Proc Natl Acad Sci U S A. 2011;108(41):16876–16882. doi: 10.1073/pnas.1114228108. Although unrelated to islet transplantation, this study demonstrates that GLP-1 promotes islet viability by activating mTOR pathway and accumulation of HIF-1a. Rapamycin, a common immunosuppressant used for islet transplant, disrupts GLP-1 beneficial effects on beta cell and may explain lacking long-term trophic effect of GLP-1 in clinical setting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Velmurugan K, Balamurugan AN, Loganathan G, et al. Antiapoptotic actions of exendin-4 against hypoxia and cytokines are augmented by CREB. Endocrinology. 2012;153(3):1116–1128. doi: 10.1210/en.2011-1895. This study shows that a combination of exendin-4 and overexpression of viral CREB exerted enhanced antiapoptotic action in cultured islets against hypoxia and cytokines. More significantly, transplantation of human islets transduced with adenoviral CREB and treated with exendin-4 showed improved glycemic control over a 30-d period in diabetic athymic nude mice. These observations have significant implications in the therapeutic potential of exendin-4 and CREB in the islet transplantation setting as well as in preservingβ-cell mass of diabetic patients. [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Perry T, Kindy MS, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106(4):1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Denker PS, Dimarco PE. Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care. 2006;29(2):471. doi: 10.2337/diacare.29.02.06.dc05-2043. [DOI] [PubMed] [Google Scholar]
- 70.Cure P, Pileggi A, Alejandro R. Exenatide and rare adverse events. N Engl J Med. 2008;358(18):1969–1970. doi: 10.1056/NEJMc0707137. discussion 1971-196. [DOI] [PubMed] [Google Scholar]
- 71.Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141(1):150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gier B, Butler PC, Lai CK, et al. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab. 2012;97(1):121–131. doi: 10.1210/jc.2011-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151(4):1473–1486. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- 74.Tatarkiewicz K, Belanger P, Gu G, et al. No evidence of drug-induced pancreatitis in rats treated with exenatide for 13 weeks. Diabetes Obes Metab. 2012 doi: 10.1111/dom.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012;98(2):271–284. doi: 10.1016/j.diabres.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 76.Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked Expansion of Exocrine and Endocrine Pancreas with Incretin Therapy in Humans with increased Exocrine Pancreas Dysplasia and the potential for Glucagon-producing Neuroendocrine Tumors. Diabetes. 2013 doi: 10.2337/db12-1686. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


