Figure 1.
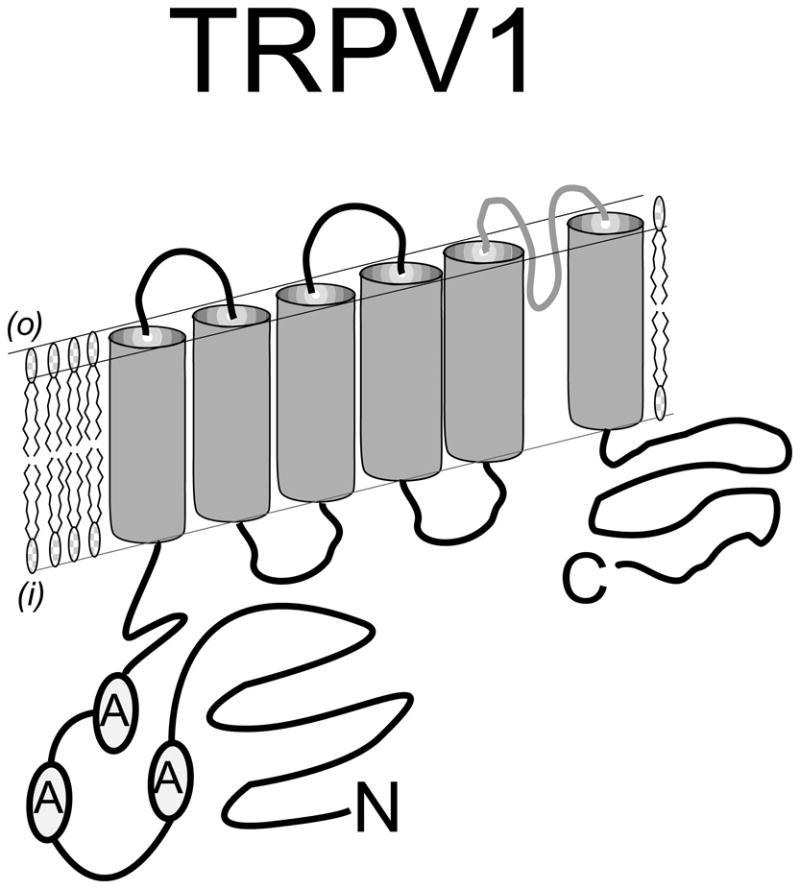
Predicted topology of a TRPV1 protein subunit spanning the plasma membrane. The N - terminal intracellular region is distinguished by three ankyrin repeat domains that participate in channel stability, activation and modulation. The C-terminal intracellular region also plays a critical role in channel activation – especially through interaction with second messenger pathways such as PKC. Six transmembrane spanning domains establish the channel structure with a pore – loop region interdicted between transmembrane domains five and six. The simplest model proposes that four of these subunits assemble to form a channel complex that is activated by the binding of capsaicin to the complex of intracellular domains.
