Abstract
Background
Although heparin is used to anticoagulate the extracorporeal circuit for most patients on maintenance hemodialysis (HD), some patients undergo heparin-free HD. We describe the determinants of heparin-free HD and its association with adverse outcomes using data from a national dialysis provider merged with Medicare claims.
Methods
We identified patients aged ≥67 years with no recent history of warfarin use who initiated maintenance HD from 2007 to 2008. We applied the Cox regression to a propensity score-matched cohort to estimate the hazards of all-cause mortality, bleeding (gastrointestinal hemorrhage, hemorrhagic stroke, other hemorrhage), atherothrombosis (ischemic stroke, myocardial infarction) and venous thromboembolism (VTE) (deep vein thrombosis, pulmonary embolism).
Results
Among 12 468 patients, 836 (6.7%) were dialyzed heparin-free. In multivariable-adjusted analyses, a history of gastrointestinal bleeding, hemorrhagic stroke and lower hemoglobin and platelet counts were associated with higher odds of heparin-free HD. Heparin-free HD use also varied as much as 4-fold by facility region. We found no significant association of heparin-free HD with all-cause mortality [hazard ratio (HR) 1.08; 95% confidence interval (CI): 0.94–1.26], bleeding (HR 1.15; 95% CI: 0.83–1.60), atherothrombosis (HR 1.09, 95% CI: 0.90–1.31) or VTE (HR 1.23, 95% CI: 0.93–1.64) compared with HD with heparin.
Conclusions
Patient markers of increased risk of bleeding and facility region associated with heparin-free HD use. Despite the potential benefits of avoiding heparin use, heparin-free HD was not significantly associated with decreased hazards of death, bleeding or thrombosis, suggesting that it may be no safer than HD with heparin.
Keywords: anti-coagulation, gastrointestinal bleeding, hemodialysis, heparin, heparin-free
INTRODUCTION
Anticoagulation facilitates hemodialysis (HD) by preventing blood clots from forming in the dialysis filter and extracorporeal tubing. In the USA, unfractionated heparin remains the most commonly used anticoagulant during maintenance HD because it is widely available at a relatively low cost and familiar to healthcare practitioners [1]. However, heparin can increase the risk of bleeding. Patients with end-stage renal disease (ESRD) requiring dialysis are already at an increased risk of bleeding as uremia can cause platelet dysfunction, and many patients on HD use anticoagulants and platelet inhibitors due to the high rate of occlusive vascular events such as vascular access failure, myocardial infarction and ischemic stroke [2–4]. Moreover, patients on HD frequently undergo invasive surgical procedures that may be complicated by hemorrhage [5]. All of these factors contribute to the high incidence of major bleeding events in patients on HD [6].
Receipt of maintenance HD without heparin, or heparin-free HD, is an alternative for patients with a contraindication to heparin use. Instead of using heparin, technicians intermittently flush the extracorporeal circuit with 100–200 mL of saline to prevent blood clots. Practitioners must weigh the decreased risk of bleeding against the increased risk of clotting in the circuit, which not only reduces dialysis efficiency, but also often requires a change of the circuit leading to extra blood loss for patients and increased time, labor and expense for providers [7–11].
However, little is known about the practice patterns and safety of the use of heparin-free HD. In this study, we aimed to characterize the use of heparin-free HD and identify clinical determinants of its use in older patients initiating HD with a national dialysis provider. We also investigated whether the use of heparin-free HD was associated with a decreased risk of mortality or bleeding, and an increased risk of atherothrombosis or venous thromboembolism (VTE).
MATERIALS AND METHODS
Data source
We used data merged from the United States Renal Data System (USRDS) and the electronic medical records (EMR) of a national dialysis provider using a crosswalk of anonymized patient identifiers that the USRDS Center generated upon approval by the Centers for Medicare and Medicaid Services (CMS) and the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK). The USRDS contains demographic information; billing claims to Medicare (Part A and B), which are available for the vast majority of Americans from the age of 65; as well as comorbidities and facility information from forms submitted to the CMS. The EMR includes records on heparin and warfarin use, vital signs, laboratory measurements and HD prescriptions.
Study population
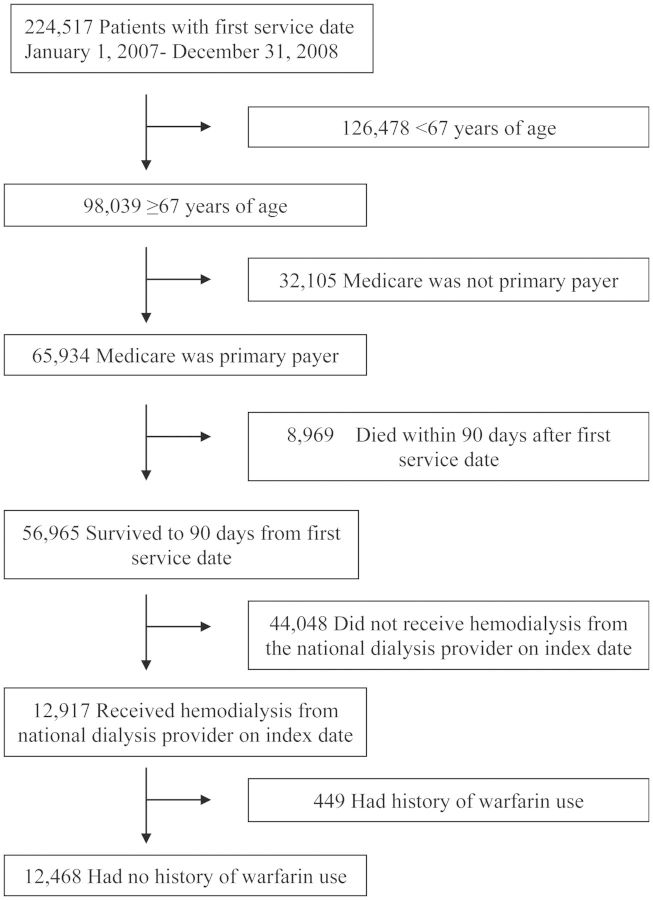
We selected a contemporary cohort of patients who initiated renal replacement therapy between 1 January 2007 and 31 December 2008 and underwent HD at a participating facility on Day 90 (±10 days; index date) after their first ESRD service date. We restricted our study population to patients 67 years of age or older whose primary payer was Medicare for at least 1 year prior to the index date so that we could uniformly ascertain comorbidities from Medicare claims data. We also excluded patients with a history of warfarin use in the year prior to the index date as the drug has been shown to increase the risk of hemorrhage in patients on dialysis [6, 12–14]. Out of the 224 517 patients in the USRDS who initiated renal replacement therapy from 2007 to 2008, 98 039 were at least 67 years of age (Figure 1). Of these patients, 65 934 had Medicare as their primary payer and 56 965 survived to 90 days from first dialysis treatment. When we restricted the patients to those without a recent history of warfarin use who received HD from the national dialysis provider on Day 90 after initiation, our final cohort included 12 468 patients.
FIGURE 1:
Study population selection from the USRDS. We selected a contemporary cohort of patients 67 years of age or older with no history of warfarin use in the past year whose primary payer was Medicare who initiated renal replacement therapy between 1 January 2007 and 31 December 2008 and underwent HD at a participating facility on Day 90 (±10 days; index date) after their first ESRD service date.
Heparin-free HD
Receipt of heparin-free HD (versus maintenance HD with heparin, any dose) served as the outcome for the first aim of our study, to identify clinical correlates of heparin-free HD use. It served as the exposure for the second aim of our study, to define the association between heparin-free HD and subsequent bleeding, thrombosis and death. The use of heparin-free HD was assessed on the index date, defined as Day 90 (±10 days) after a patient's first ESRD service date. We chose this index date assuming that any heparin regimen would have been titrated and stable by this time. We abstracted data on both the treatment date and heparin administration from the EMR of the dialysis provider. In order to assess whether the treatment exposure observed on the index date represented a more consistent practice (or prescription) for any given patient, we validated the exposure of interest by examining the use of heparin versus heparin-free HD 1 week (7 ± 2 days), 1 month (28 ± 7 days), 6 months (182 ± 7 days) and 1 year (364 ± 7 days), after the index treatment.
Death, bleeding events, atherothrombosis and VTE
To evaluate the safety of heparin-free HD, we evaluated its association with clinically important adverse outcomes, including death, bleeding events, atherothrombosis and VTE. Events were ascertained from inpatient Medicare claims data and the USRDS death file (Supplementary data, Table S1) based on previously validated algorithms where available, in combination with a comprehensive search of the USRDS death file cause of death and ICD-9 diagnosis codes [13, 15–18]. Bleeding events included gastrointestinal hemorrhage, hemorrhagic stroke and other hemorrhage. Atherothrombotic events included ischemic stroke and myocardial infarction, and VTE included deep vein thrombosis and pulmonary embolism. We followed patients until 1 January 2010.
Other variables
Demographics, comorbidities, vital signs, laboratory measurements, dialysis characteristics and facility factors were analyzed as potential correlates of heparin-free HD. All were chosen a priori as potentially clinically relevant determinants.
Demographic factors were obtained from the USRDS and included age on the index date, sex, race (white, black, or other) and Hispanic ethnicity. Forty (0.3%) patients with missing Hispanic ethnicity data were considered non-Hispanic.
A list of the comorbidities examined and their definitions are provided in Supplementary data, Table S2. Comorbidity status was derived from both the Medical Evidence Report (CMS form 2728) and Medicare claims data predating the index date by up to 1 year. We used previously validated algorithms where available in combination with a comprehensive search of ICD-9 diagnosis codes to define comorbidities based on Medicare claims data; comorbidities were assigned if coded in at least one inpatient claim or two outpatient claims more than 30 days apart in the year prior to the index date [16].
Vital signs and laboratory measurements were abstracted from the EMR. Vital signs were taken on the index date and included pre-dialysis systolic blood pressure [in mmHg: <120, 120–139 (referent), 140–159 or ≥160] and prescribed dry weight [in kg by quartile: <61 (referent), 61–71.4, 71.5–83.5 and >83.5]. The most recent laboratory measurements drawn in the 90 days prior to the index date were analyzed and included hemoglobin level [in g/dL: <9, 9–9.9, 10–10.9, 11–11.9 (referent), 12–12.9, 13–13.9 and ≥14]; platelet count [in 103/µL: <149, 150–229, 230–310 (referent), 311–400 and >400] and albumin concentration [in g/dL: <2.5, 2.5–2.9, 3–3.4, 3.5–3.9 (referent) and ≥4].
Dialysis characteristics were abstracted from the EMR and included vascular access type, prescribed length of the dialysis treatment, reuse status of the dialyzer and Kt/V. The primary access type was categorized as central venous catheter, fistula or graft (referent) and unknown. The prescribed duration of the treatment given on the index date was divided into the following categories (in minutes): <180, 180–194, 195–209, 210–224 (referent), 225–239 and ≥240. Reuse status of the dialyzer was categorized as ever versus never reused. The most recent Kt/V in the 90 days prior to the index date was abstracted.
Facility factors were taken from the ESRD Facility Survey (form CMS-2744) conducted in the year a patient initiated dialysis, and included size of the dialysis unit, rural versus urban status and US Census Bureau Divisions. Size was defined as the number of outpatient HD patients treated at a center at the end of the survey period, and categorized by quartile [<53 (referent), 53–75, 76–106 and ≥107]. Facilities were considered urban if they were classified as a metropolitan area in the Rural–Urban Commuting Area (RUCA) Codes version 2.0, which are based on 2000 Census commuting data and 2004 zip codes; all other areas were considered to be rural [19]. Facilities were categorized into one of the nine US Census Bureau Divisions based on their state. We classified one facility in Puerto Rico and one facility in the Virgin Islands as South Atlantic as these territories are not part of any defined census division [20].
Statistical analysis
We described baseline characteristics of the cohort using means and standard deviations for normally distributed continuous data and counts and proportions for categorical data.
We performed unadjusted and adjusted analyses of potential correlates of heparin-free HD using logistic regression. As the outcome was rare (<10%), the odds ratio approximates the risk ratio, or relative risk. For the multivariable model, we included all of the variables listed in Table 2. Of note, we excluded heparin-induced thrombocytopenia as there were only two patients in the cohort coded with this condition. We only analyzed subjects without any missing information on the study variables and did not impute any missing data. All statistical tests were two-tailed. P-values of <0.05 were considered statistically significant, and we did not adjust for multiple comparisons.
Table 2:
Correlates of heparin-free maintenance HD
| Variable | Unadjusted ORa (95%CI) | Multivariable OR (95% CI) |
|---|---|---|
| Patient characteristics | ||
| Demographics | ||
| Age (by 10 year) | 1.14 (1.03–1.28) | 0.93 (0.82–1.06) |
| Sex (male versus female) | 0.89 (0.77–1.03) | 0.95 (0.80–1.13) |
| Race | ||
| Black | 0.84 (0.71–1.00) | 0.72 (0.58–0.89) |
| White | Reference | Reference |
| Other | 1.08 (0.78–1.49) | 0.95 (0.64–1.41) |
| Hispanic ethnicity | 0.87 (0.67–1.13) | 1.00 (0.73–1.37) |
| Reported comorbidities | ||
| Arrhythmia | 1.33 (1.14–1.55) | 1.15 (0.97–1.37) |
| Cancer | 1.24 (1.04–1.50) | 1.04 (0.84–1.28) |
| Coronary artery disease | 1.15 (1.00–1.33) | 1.01 (0.85–1.20) |
| Deep vein thrombosis | 1.79 (1.44–2.22) | 1.42 (1.10–1.83) |
| Diabetes mellitus | 0.80 (0.70–0.93) | 0.91 (0.77–1.07) |
| Gastrointestinal bleeding | 1.72 (1.43–2.07) | 1.42 (1.15–1.75) |
| Heart failure | 1.21 (1.05–1.40) | 1.15 (0.97–1.36) |
| Hemorrhagic stroke | 3.11 (1.97–4.90) | 2.96 (1.78–4.93) |
| Ischemic stroke | 1.12 (0.94–1.34) | 1.03 (0.84–1.26) |
| Liver disease | 1.67 (1.25–2.25) | 1.11 (0.79–1.57) |
| Peripheral vascular disease | 0.94 (0.81–1.10) | 0.81 (0.68–0.97) |
| Pulmonary disease | 1.14 (0.97–1.33) | 1.00 (0.84–1.21) |
| Pulmonary embolism | 1.45 (0.91–2.32) | 0.91 (0.51–1.63) |
| Vital signs and laboratory measurements | ||
| Weight (kg) by quartile | P for trend <0.001 | P for trend <0.001 |
| <61 | Reference | Reference |
| 61–71.4 | 0.78 (0.64–0.94) | 0.77 (0.62–0.96) |
| 71.5–83.5 | 0.71 (0.59–0.86) | 0.74 (0.59–0.93) |
| >83.5 | 0.50 (0.40–0.62) | 0.52 (0.40–0.68) |
| Pre-dialysis systolic blood pressure (mmHg) | P for trend <0.001 | P for trend = 0.01 |
| <120 | 1.27 (1.05–1.54) | 1.08 (0.87–1.35) |
| 120–139 | Reference | Reference |
| 140–159 | 0.70 (0.57–0.85) | 0.80 (0.65–1.00) |
| ≥160 | 0.69 (0.57–0.84) | 0.86 (0.69–1.07) |
| Hemoglobin (g/dL) | P for trend <0.001 | P for trend <0.001 |
| <9 | 2.43 (1.74–3.41) | 2.09 (1.42–3.07) |
| 9–9.9 | 1.94 (1.47–2.58) | 1.85 (1.35–2.54) |
| 10–10.9 | 1.42 (1.12–1.79) | 1.31 (1.02–1.69) |
| 11–11.9 | Reference | Reference |
| 12–12.9 | 0.72 (0.59–0.89) | 0.75 (0.60–0.93) |
| 13–13.9 | 0.44 (0.35–0.57) | 0.46 (0.35–0.59) |
| ≥14 | 0.24 (0.17–0.34) | 0.26 (0.18–0.37) |
| Platelets (×103/µL) | P for trend <0.001 | P for trend <0.001 |
| <150 | 3.71 (3.01–4.58) | 3.65 (2.91–4.57) |
| 150–229 | 1.46 (1.20–1.77) | 1.55 (1.26–1.91) |
| 230–310 | Reference | Reference |
| 311–400 | 0.98 (0.73–1.31) | 0.84 (0.61–1.15) |
| >400 | 1.21 (0.83–1.77) | 0.73 (0.48–1.12) |
| Albumin (g/dL) | P for trend <0.001 | P for trend = 0.44 |
| <2.5 | 1.87 (1.30–2.69) | 1.02 (0.67–1.56) |
| 2.5–2.9 | 1.73 (1.37–2.19) | 1.13 (0.87–1.48) |
| 3–3.4 | 1.36 (1.14–1.62) | 1.17 (0.97–1.42) |
| 3.5–3.9 | Reference | Reference |
| ≥4 | 0.98 (0.78–1.22) | 1.11 (0.88–1.40) |
| Dialysis characteristics | ||
| Vascular access used during index HD | ||
| Central venous catheter | 0.97 (0.83–1.13) | 0.76 (0.64–0.90) |
| Fistula or graft | Reference | Reference |
| Length of session (min) | P for trend <0.001 | P for trend = 0.02 |
| <180 | 2.03 (1.44–2.86) | 1.98 (1.32–2.98) |
| 180–194 | 1.12 (0.94–1.34) | 1.09 (0.89–1.34) |
| 195–209 | 1.05 (0.79–1.41) | 1.07 (0.78–1.46) |
| 210–224 | Reference | Reference |
| 225–239 | 0.82 (0.59–1.14) | 0.95 (0.67–1.35) |
| ≥240 | 0.84 (0.69–1.03) | 0.95 (0.76–1.18) |
| Reuse of dialysis filter (any) | 0.39 (0.33–0.45) | 0.41 (0.35–0.49) |
| Facility characteristics | ||
| Number of outpatient hemodialysis patients (by quartile) | P for trend <0.001 | P for trend = 0.04 |
| <53 | Reference | Reference |
| 53–75 | 1.11 (0.90–1.37) | 1.04 (0.82–1.32) |
| 76–106 | 1.27 (1.03–1.57) | 1.13 (0.89–1.43) |
| ≥107 | 1.43 (1.17–1.76) | 1.26 (0.98–1.61) |
| Rural (versus urban) | 0.64 (0.52–0.79) | 0.81 (0.65–1.03) |
| Census division | P(Wald) <0.001 | P(Wald) <0.001 |
| East North Central | 0.85 (0.68–1.07) | 0.85 (0.66–1.09) |
| East South Central | 0.60 (0.41–0.89) | 0.59 (0.39–0.89) |
| Middle Atlantic | 1.64 (1.32–2.04) | 1.11 (0.87–1.42) |
| Mountain | 0.83 (0.58–1.20) | 0.77 (0.52–1.14) |
| New England | 2.07 (1.55–2.75) | 1.51 (1.11–2.06) |
| Pacific | 0.82 (0.65–1.04) | 0.71 (0.53–0.95) |
| South Atlantic | Reference | Reference |
| West North Central | 0.40 (0.26–0.62) | 0.37 (0.23–0.61) |
| West South Central | 0.58 (0.43–0.78) | 0.51 (0.36–0.71) |
an for multivariable analysis = 11 632: 756 recipients of heparin-free HD and 10 876 recipients of heparin with HD. Odds ratios (OR) and 95% CIs.
Given the observational nature of our study, and the inherent risk of confounding by indication, we conducted a propensity score-matched survival analysis. Using the same multivariable logistic regression model described above, we generated propensity scores for the use of heparin-free HD. We applied a greedy-match algorithm to match 1:1 heparin-free HD users with non-users with a maximum difference in the propensity score of 0.01. Matches were only allowed within the same census division. Differences between the two exposure groups in the matched cohort were compared using standardized differences, with a value of <10% representing adequate balance in the distribution of the variable between the two groups [21]. Proportional hazards regression was applied to this matched cohort to estimate the hazard ratio (HR) for death in heparin-free HD users versus non-users. We censored patients for kidney transplantation and end of the follow-up period (1 January 2010). Violation of the proportional hazards assumption was checked using interaction terms with time.
Proportional hazards regression was applied to the same propensity score-matched cohort to estimate the HR for bleeding events in heparin-free HD users versus non-users. As patients may have had multiple events, we only analyzed the first event they experienced. We censored patients for kidney transplantation, death not related to bleeding and the end of the follow-up period. Analogous analyses were conducted to estimate the HRs for atherothrombosis and VTE.
All analyses were performed using SAS Enterprise Guide 4.3 (SAS Institute Inc., Cary, NC).
Sensitivity analyses
Given the potential for confounding in this observational study, we performed a number of sensitivity analyses for the survival analysis. We estimated an unadjusted HR for death using the full cohort as well as an HR adjusted for all of the variables included in the propensity score model. We also performed an analysis on the full cohort stratified by quintile of propensity score. Finally, we also analyzed the propensity score-matched cohort but censored patients for a change in exposure.
RESULTS
Patient characteristics
Out of 12 468 patients, 836 (6.7%) did not receive any heparin during their index HD treatment. Table 1 shows the baseline characteristics of patients, assessed on or in the year prior to their index date. Patients undergoing heparin-free HD compared with standard HD (with heparin) had similar mean age, proportion of women and racial/ethnic distribution. However, patients undergoing heparin-free HD had a higher prevalence of several comorbid conditions, with the notable exception of diabetes mellitus. Patients receiving heparin-free HD had lower baseline weight, lower systolic blood pressure, hemoglobin level, platelet count and serum albumin concentration.
Table 1.
Baseline characteristics of older patients receiving maintenance HD, ∼90 days after initiation, before and after matching on propensity score
| Variable | Full cohort |
Propensity score-matched cohort |
||||
|---|---|---|---|---|---|---|
| Recipients of heparin-free hemodialysis (n = 836) | Recipients of heparin with hemodialysis (n = 11 632) | Std. diff (%) | Recipients of heparin-free hemodialysis (n = 728) | Recipients of heparin with hemodialysis (n = 728) | Std. diff (%) | |
| Demographics | ||||||
| Age (year, mean ± SD) | 77 ± 6 | 76 ± 6 | 8.5 | 77 ± 6 | 77 ± 7 | 8.3 |
| Male sex, n (%) | 421 (50) | 6194 (53) | 5.7 | 374 (51) | 370 (51) | 1.1 |
| Race, n (%) | ||||||
| Black | 164 (20) | 2622 (23) | 7.2 | 143 (20) | 143 (20) | 0 |
| White | 630 (75) | 8484 (73) | 5.5 | 554 (76) | 546 (75) | 2.6 |
| Other | 42 (5) | 526 (5) | 2.3 | 31 (4) | 39 (5) | 5.1 |
| Hispanic ethnicity | 63 (8) | 997 (9) | 3.8 | 55 (8) | 57 (8) | 1 |
| Reported comorbidities, n (%) | ||||||
| Arrhythmia | 266 (32) | 3022 (26) | 12.9 | 228 (31) | 230 (32) | 0.6 |
| Cancer | 149 (18) | 1727 (15) | 8.1 | 130 (18) | 122 (17) | 2.9 |
| Coronary artery disease | 329 (39) | 4186 (36) | 7.0 | 283 (39) | 274 (38) | 2.5 |
| Deep vein thrombosis | 106 (13) | 873 (8) | 17.2 | 88 (12) | 79 (11) | 3.9 |
| Diabetes mellitus | 424 (51) | 6529 (56) | 10.9 | 373 (51) | 372 (51) | 0.3 |
| Gastrointestinal bleeding | 154 (18) | 1349 (12) | 19.2 | 129 (18) | 120 (16) | 3.3 |
| Heart failure | 499 (60) | 6392 (55) | 9.5 | 431 (59) | 415 (57) | 4.5 |
| Hemorrhagic stroke | 23 (3) | 105 (1) | 13.8 | 18 (2) | 21 (3) | 2.6 |
| Heparin-induced thrombocytopeniaa | −(<1) | −(<1) | 4.4 | −(<1) | −(<1) | 5.2 |
| Ischemic stroke | 164 (20) | 2076 (18) | 4.6 | 141 (19) | 140 (19) | 0.3 |
| Liver disease | 53 (6) | 452 (4) | 11.2 | 37 (5) | 37 (5) | 0 |
| Peripheral vascular disease | 247 (30) | 3592 (31) | 2.9 | 208 (29) | 211 (29) | 0.9 |
| Pulmonary disease | 224 (27) | 2836 (24) | 5.5 | 184 (25) | 166 (23) | 5.8 |
| Pulmonary embolism | 20 (2) | 193 (2) | 5.2 | 15 (2) | 17 (2) | 1.9 |
| Vital signs and laboratory measurements (mean ± SD) | ||||||
| Weight (kg) | 69 ± 16 | 74 ± 18 | 26.6 | 70 ± 16 | 70 ± 18 | 2.3 |
| Pre-dialysis systolic blood pressure (mmHg) | 139 ± 28 | 145 ± 27 | 23.0 | 140 ± 27 | 140 ± 27 | 0.7 |
| Hemoglobin (g/dL) | 11.6 ± 1.6 | 12.5 ± 1.5 | 57.6 | 11.7 ± 1.6 | 11.7 ± 1.6 | 3.6 |
| Platelet count (×103/µL) | 205 ± 101 | 240 ± 91 | 37.1 | 207 ± 97 | 215 ± 99 | 8.3 |
| Albumin (g/dL) | 3.4 ± 0.5 | 3.5 ± 0.5 | 21.5 | 3.4 ± 0.5 | 3.4 ± 0.5 | 1.9 |
| Dialysis characteristics | ||||||
| Kt/V | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.3 | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.9 |
| Vascular access, n (%) | ||||||
| Central venous catheter | 589 (70) | 8282 (71) | 1.6 | 504 (69) | 510 (70) | 1.8 |
| Fistula or graft | 239 (29) | 3255 (28) | 1.3 | 218 (30) | 213 (29) | 1.5 |
| Length of session (min, mean ± SD) | 205 ± 26 | 209 ± 25 | 15.1 | 206 ± 25 | 206 ± 24 | 0.1 |
| Reuse | 266 (32) | 6347 (55) | 47.1 | 251 (34) | 234 (32) | 5.0 |
| Facility characteristics | ||||||
| Number of outpatient hemodialysis patients (mean ± SD) | 88 ± 43 | 83 ± 44 | 11.2 | 87 ± 41 | 88 ± 42 | 1.9 |
| Rural, n (%) | 112 (13) | 2281 (20) | 16.4 | 101 (14) | 94 (13) | 2.8 |
| Census division, n (%)b | ||||||
| East North Central | 118 (14) | 1847 (16) | 4.9 | 108 (15) | 108 (15) | 0 |
| East South Central | 30 (4) | 663 (6) | 10.0 | 27 (4) | 27 (4) | 0 |
| Middle Atlantic | 144 (17) | 1172 (10) | 20.1 | 130 (18) | 130 (18) | 0 |
| Mountain | 35 (4) | 559 (5) | 3.0 | 31 (4) | 31 (4) | 0 |
| New England | 70 (8) | 452 (4) | 18.8 | 64 (9) | 64 (9) | 0 |
| Pacific | 102 (12) | 1656 (14) | 6.0 | 88 (12) | 88 (12) | 0 |
| South Atlantic | 232 (28) | 3092 (27) | 2.6 | 215 (30) | 215 (30) | 0 |
| West North Central | 22 (3) | 734 (6) | 17.9 | 18 (2) | 18 (2) | 0 |
| West South Central | 54 (6) | 1244 (11) | 15.1 | 47 (6) | 47 (6) | 0 |
SD, standard deviation; Std. diff, standardized difference.
Variables and the n (%) recipients of heparin-free HD versus n (%) recipients of heparin with HD missing data in the unmatched sample: weight: 34 (4) versus 323 (3); pre-dialysis systolic blood pressurea: −(1) versus 32 (<1); hemoglobin: 34 (4) versus 215 (2); platelet count: 38 (5) versus 271 (2); albumin: 38 (5) versus 236 (2); Kt/V: 62 (7) versus 480 (4); vascular accessa: −(1) versus 95 (1); length of sessiona: −(<1) versus −(<1); facility number of outpatient HD patients: 29 (3) versus 242 (2); facility rural status: 29 (3) versus 237 (2); facility census division: 29 (3) versus 213 (2).
aPer federal research regulations, any cell counts <10 must not be reported. This variable was not included in the estimation of the propensity score, but was exactly balanced in the propensity score-matched cohort.
bPairs were matched on propensity score within each Census division (standardized differences are zero by default).
The dialysis treatment characteristics of the two groups varied as well. Both groups had a high rate of catheter use (71%) as their primary vascular access. However, recipients of heparin-free HD dialyzed for slightly shorter periods of time (mean 205 versus 209 min) and were nearly half as likely to have reused their dialyzer than patients administered heparin during HD (32 versus 55%). Yet, their Kt/V was no different from those who dialyzed with heparin.
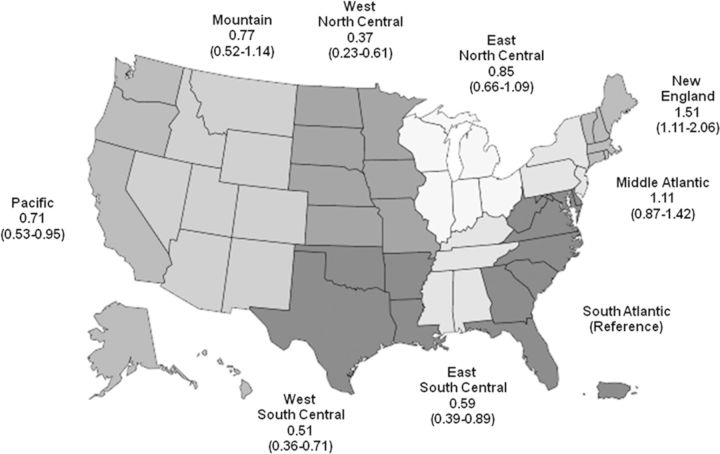
We also observed marked differences between the groups by facility factors. Recipients of heparin-free HD dialyzed in slightly larger centers (88 versus 83 point prevalent outpatient HD patients). Fewer recipients of heparin-free HD underwent treatment in rural centers (13 versus 20%). Heparin-free HD was relatively more common in the New England and Middle Atlantic census divisions: nearly twice as many recipients of heparin-free HD as recipients of standard HD dialyzed in these two divisions (26 versus 14%)
Correlates of heparin-free HD
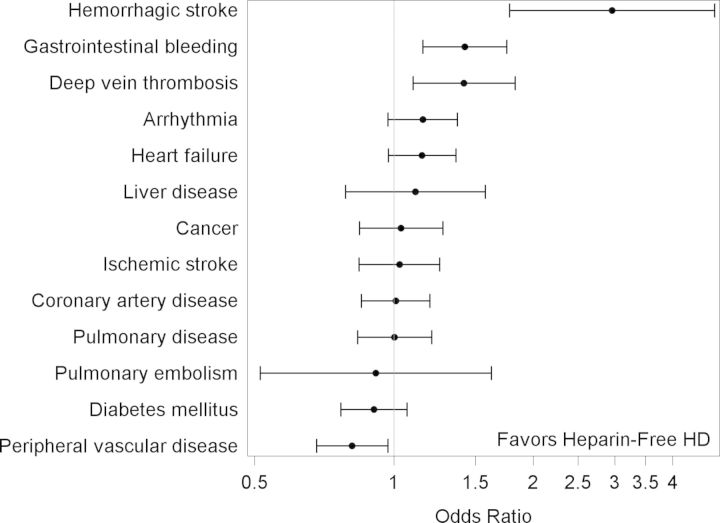
Results of the unadjusted analyses are shown in Table 2. After multivariable adjustment for all of the variables listed in Table 2, black race (versus white race) was the only demographic characteristic independently associated with lower odds of receiving heparin-free HD (Table 2). A number of comorbidities remained independently associated with the use of heparin-free HD, including a history of deep vein thrombosis, gastrointestinal bleeding and hemorrhagic stroke (Figure 2). Lower weight, systolic blood pressure, hemoglobin level and platelet count, as well as shorter duration of the HD session were also all associated with a higher likelihood of heparin-free HD (Figure 3). In contrast, patients who had a central venous catheter as their vascular access (versus fistula or graft) or whose dialysis filter was reused were less likely to dialyze heparin-free. There was regional variation in the use of heparin-free HD as well, with patients in New England more than four times as likely to use heparin-free HD as patients in West North Central (Figure 4).
FIGURE 2:
Multivariable odds ratios and 95% CIs of receiving heparin-free maintenance HD among older incident patients, by comorbidity. X-axis is on the log scale.
FIGURE 3:
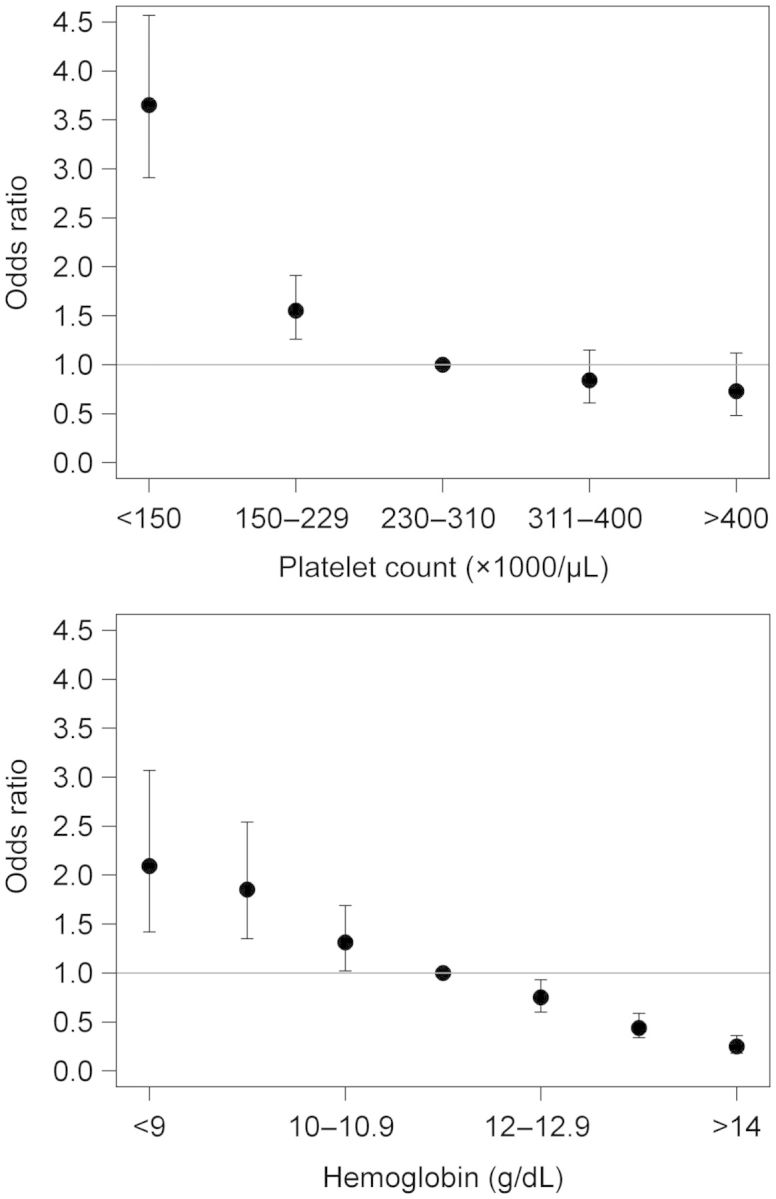
Multivariable odds ratios and 95% CIs of receiving heparin-free maintenance HD among older incident patients, by platelet count (top) and hemoglobin level (bottom). Ptrend < 0.001 for both parameters.
FIGURE 4:
Multivariable odds ratios and 95% CIs of receiving heparin-free maintenance HD among older incident patients, by census division. The South Atlantic census division was selected as the reference group as it contained the most patients.
Association of heparin-free HD with adverse outcomes
The c-statistic for the propensity score model was 0.78, indicating good prediction. We matched on their propensity scores 728 recipients (87%) of heparin-free HD with 728 recipients of heparin with HD. All characteristics were balanced between the two groups in the matched cohort, as demonstrated by standardized differences <10% (Table 1) [22]. Additionally, the matched heparin-free HD recipients were similar to the overall cohort of heparin-free HD recipients.
Over 1763 person-years of follow-up, we observed 702 deaths for a mortality rate of 40 deaths per 100 person-years (Table 3). We found no association between the use of heparin-free HD (versus use of heparin with HD) and mortality [HR = 1.08, 95% confidence interval (CI): 0.94–1.26].
Table 3:
Number of events, follow-up time, incidence rates and HRs for all study outcomes based on propensity score-matched cohorta
| Outcome | Exposure group | Number of events | Follow-up time (years) |
Incidence rate (per 100 person-years) | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Mean ± SD | Median | |||||
| All bleeding events | Heparin-free | 75 | 1.14 ± 0.76 | 1.07 | 9.1 | 1.15 (0.83–1.60) |
| Heparin | 67 | 1.18 ± 0.77 | 1.10 | 7.8 | ||
| Atherothrombotic eventsb | Heparin-free | 228 | 1.08 ± 0.76 | 0.99 | 28.9 | 1.09 (0.90–1.31) |
| Heparin | 217 | 1.13 ± 0.77 | 1.02 | 26.5 | ||
| Venous thromboembolismsc | Heparin-free | 102 | 1.08 ± 0.76 | 0.98 | 12.9 | 1.23 (0.93–1.64) |
| Heparin | 87 | 1.15 ± 0.77 | 1.03 | 10.4 | ||
| All-cause mortality | Heparin-free | 360 | 1.19 ± 0.77 | 1.16 | 41.5 | 1.08 (0.94–1.26) |
| Heparin | 342 | 1.23 ± 0.77 | 1.17 | 38.1 | ||
SD, standard deviation; CI, confidence interval.
an = 728 in the heparin group and 728 in the heparin-free group.
bAtherothrombotic events included myocardial infarction and ischemic stroke.
cVTEs included deep vein thrombosis and pulmonary embolism.
When analyzing bleeding events, we observed a rate of 8.4 bleeding events per 100 person-years, which did not differ significantly from the HD with the heparin group (HR = 1.15, 95% CI: 0.83–1.60). Similarly, the rates of both atherothrombosis (HR = 1.09, 95% CI: 0.90–1.31) and VTE (HR = 1.23, 95% CI: 0.93–1.64) did not differ significantly by heparin use (Table 3).
Results from the sensitivity analyses are shown in Table 4 and were comparable to the primary analysis. Although the unadjusted HR for death in the full cohort was significantly >1 (HR = 1.41, 95% CI: 1.27–1.55), the results were attenuated when adjusted for patient, dialysis and facility characteristics (HR = 1.06, 95% CI: 0.95–1.19). Stratifying by quintile of propensity score yielded similar results to the adjusted analysis (HR = 1.12, 95% CI: 1.00–1.25). When we restricted the population to the propensity score-matched cohort and further censored for change in exposure to heparin-free HD, the HR was higher than that from primary analysis, but inference did not change due to confidence limits that included the null value (HR = 1.18, 95% CI: 0.92–1.52).
Table 4:
HRs for death for recipients of heparin-free HD based on various statistical models
| Model | Hazard ratio (95% confidence interval) |
|---|---|
| Full cohort, unadjusted | 1.41 (1.27–1.55) |
| Full cohort, adjusted | 1.06 (0.95–1.19) |
| Full cohort, stratified by quintile of propensity score | 1.12 (1.00–1.25) |
| Propensity score-matched cohort | 1.08 (0.94–1.26) |
| Propensity score-matched cohort, censored for change in exposure | 1.18 (0.92–1.52) |
Consistency over time of baseline heparin use during HD
To examine whether the use of heparin-free HD versus use of heparin with HD represented a longer care pattern rather than a one-time occurrence, we also assessed these practices during the HD sessions 1 week, 1 month, 6 months and 1 year after the index date in the propensity score-matched cohort. Practices remained consistent within a month of the index date. Of those who received heparin-free HD on the index date, 91 and 81% continued to dialyze heparin-free 1 week and 1 month after the index date, respectively (Table 5). These percentages continued to drop, though, and by 1 year after the index date, only about half of heparin-free recipients were still dialyzing without the drug. In contrast, of those who received heparin during their index treatment, 99% were still receiving it during their HD sessions a week after the index date. This percentage dropped slightly to 91% a year after the index date. These data are conditional on patients receiving HD at a participating unit on those follow-up days. Thus, while heparin users remain consistently on the drug, heparin-free HD recipients are more likely to start receiving heparin as time goes on.
Table 5:
Consistency of the use of heparin-free HD and of HD with heparin in the propensity score-matched cohort at 1 week, 1, 6 months and 1 year from the index date
| Date | Recipients of heparin-free hemodialysis, n (%) | Recipients of heparin with hemodialysis, n (%) |
|---|---|---|
| Index date | 728 (100%) | 728 (100%) |
| Index date + 7 (±2) days | ||
| Heparin-free hemodialysis | 638 (91%) | 7 (1%) |
| Heparin with hemodialysis | 63 (9%) | 705 (99%) |
| Index date + 28 (±7) days | ||
| Heparin-free hemodialysis | 534 (81%) | 16 (2%) |
| Heparin with hemodialysis | 122 (19%) | 656 (98%) |
| Index date + 182 (±7) days | ||
| Heparin-free hemodialysis | 306 (62%) | 33 (8%) |
| Heparin with hemodialysis | 190 (38%) | 492 (92%) |
| Index date + 364 (±7) days | ||
| Heparin-free hemodialysis | 191 (52%) | 35 (9%) |
| Heparin with hemodialysis | 175 (48%) | 338 (91%) |
n recipients of heparin-free HD versus n recipients of heparin with HD missing treatment data: index date + 7 days: 27 versus 16; index date + 28 days: 72 versus 56; index date + 182 days: 232 versus 203; index date + 364 days: 362 versus 355.
DISCUSSION
In our study of the use of heparin-free maintenance HD in the USA, we found that patients were more likely to dialyze heparin-free if they had conditions that confer or are markers for an increased risk of bleeding, including lower platelet and hemoglobin levels, and medical histories of gastrointestinal bleeding and hemorrhagic stroke. The association between a history of deep vein thrombosis and heparin-free HD is likely confounded by the prescription of anticoagulant and antiplatelet medications to treat this condition. Although we excluded patients with a recent history of warfarin use, we did not have information on aspirin or clopidogrel use. Thus, the use of these two medications may be driving the otherwise paradoxical connection between a condition associated with clotting events and heparin-free HD. Overall, our study suggests that physicians are considering patients' history when prescribing heparin-free HD, reserving the strategy for those at greatest risk of hemorrhage.
The correlation between the use of a central venous catheter (versus fistula or graft) and lower odds of dialyzing heparin-free is notable. Catheters have a high rate of malfunction, with the mean time to first thrombosis ranging from 73 to 84 days [23, 24]. While fistulas and grafts can also thrombose, management of peripheral access thrombosis usually involves surgical intervention, and is sometimes followed by the prescription of antiplatelet or anticoagulant drugs [25]. Both treatments would likely prompt practitioners to favor a heparin-free HD session for patients with a history of stenotic or thrombosed peripheral access. The mainstay of prevention and treatment of catheter malfunction, on the other hand, is catheter locks, most often with heparin [26]. If anything, practitioners should be more likely to prescribe heparin with HD to promote catheter patency, which is consistent with what we observed.
This study demonstrated that patterns of heparin-free HD use vary by geographic region, independent of a number of patient factors. Previous studies have demonstrated geographic variation in other dialysis practices, including vascular access placement, modality selection and anemia management [27–30]. Potential explanations for these findings included differences in the demographics, healthcare utilization and urbanization of the various regions. However, in our analysis, we adjusted for patient age, race, ethnicity and urbanization. Furthermore, we restricted the cohort to Medicare patients dialyzing with a specific national provider, eliminating confounding by regional variation in insurance status or choice of dialysis chain. Thus, these observations suggest that physician or facility preference or regional culture may play a role in the administration of heparin-free HD, not surprising given that there are no guidelines for its use.
We observed no difference in the risk of death for recipients of heparin-free HD when compared with recipients of heparin with HD. This result was attenuated but still consistent with the results of the sensitivity analyses, which showed a largely non-significant trend toward an increased risk of mortality for recipients of heparin-free HD. It is important to note that the analyses based on the full cohort include heparin with HD recipients who would likely never receive heparin-free HD; this population is generally less frail on observed characteristics and comorbidities than patients receiving heparin-free HD. Thus, it was expected that these analyses yielded HRs that were further from the null than the primary analysis. The propensity score-matched analysis is arguably a more accurate model as the two exposure groups were equally likely to receive heparin-free HD, based on observed characteristics.
Of note, we performed an intention-to-treat analysis, classifying exposure status based on the receipt of heparin-free HD on the index date. However, while the vast majority of heparin users remained on heparin, the longer a heparin-free recipient survived, the more likely he was to crossover and receive heparin, to the extent that almost half of heparin-free HD recipients were using the drug a year after the index date. This misclassification, though, biased the results toward the null; when we censored patients for a change in exposure, the HR increased from 1.08 to 1.18. This strengthens the conclusion that despite avoiding heparin and its potentially lethal side effects, recipients of heparin-free dialysis did not have a lower risk of death.
Although bleeding is the most common complication of heparin use, we did not find a significant association between heparin-free HD and bleeding. This is consistent with the limited data available on heparin use in HD patients [31]. In a cohort of US dialysis patients, Wasse et al. [32] found that the use of any antiplatelet or anticoagulant medication was not associated with increased risk of gastrointestinal bleeding. A Japanese study found that there was no difference in the dose of heparin given to HD patients who had experienced a hemorrhagic stroke versus those who had not [33]. The majority of data on other major bleeding events such as retroperitoneal hemorrhage, ophthalmologic bleeding and hemopericardium are limited to case series from the 1970s and 1960s, and the role heparin played in precipitating these events is unclear at best [34–41].
Our bleeding analysis was limited by a lack of data on whether a patient had previously clotted an extracorporeal circuit, which can influence the propensity to receive heparin-free HD. For instance, a patient who has a high risk of bleeding may nevertheless be given heparin if he has a history of frequent clotting of the circuit with heparin-free HD. However, the absence of these data biased our estimated HR, which was >1, toward the null. Again, this supports the notion that heparin-free HD may not be safer than heparin because recipients did not have a lower hazard of bleeding.
We also found no association between heparin-free HD and either atherothrombosis or VTE. This was not surprising, as the dosage of heparin with HD does not approach the levels recommended for thromboprophylaxis. The most common thrombotic event complicating heparin-free HD is clotting of the dialysis circuit, which can lead to inadequate dialysis if it results in the premature cessation of the session. Unfortunately, clotting of the circuit was not captured in the database. However, the average Kt/V did not differ between the two groups, so if there was a difference in the rate of circuit clots between the groups, it did not significantly affect the dose of dialysis delivered.
Our time-to-event analysis was limited by the short observation period, with a median length of follow-up of only 1.16 years. Other study limitations include the inability to analyze several potential correlates of heparin-free HD, including the use of certain medications such as aspirin or clopidogrel, the type of dialyzer membrane used, how often the membrane had been reused, whether the patient had clotted a circuit previously and the specific indication or facility-specific beliefs or protocols for heparin-free HD. Similarly, we could not adjust for activated partial thromboplastin time or international normalized ratio as they were not reliably measured in the vast majority of patients. Catheter obstruction and infection were not captured either, so we were unable to study the association of heparin with these access-related complications. Comorbidities were ascertained from administrative data, leaving the potential for misclassification of presence as well as severity of these conditions. Thus, as with all observational studies, we cannot eliminate residual confounding by indication. Finally, the population was restricted to older Medicare recipients without a recent history of warfarin use dialyzing with one particular dialysis provider, which may limit the generalizability of our study, although physicians tend to see patients with more than one provider in the area where they practice [42].
These limitations must be weighed against the strengths of the study: use of a large, national, contemporary cohort that accounted for patient, dialysis and facility characteristics, which are rarely available in such detail in the same data set.
In conclusion, our study shows that heparin-free HD is used infrequently, in <7% of patients, and correlates with patient specific conditions, including markers for increased risk of bleeding, as well as facility factors such as census division. Despite the potential benefits of avoiding heparin use, heparin-free HD was not significantly associated with a decreased risk of death, bleeding, atherothrombosis or VTE. Our study suggests that heparin-free HD may not be safer than HD with heparin, and that a randomized trial should be considered to settle this comparative effectiveness question.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
FUNDING
This work was supported by the American Kidney Fund-Amgen Clinical Scientist in Nephrology grant to J.I.S. It was also funded by grant R21DK077336 from the NIH to W.C.W.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part, except in abstract form.
Supplementary Material
ACKNOWLEDGEMENTS
J.I.S. is a 2010 American Kidney Fund-Amgen Clinical Scientist in Nephrology Fellow. This work was also supported by grant R21DK077336 from the NIDDK to W.C.W. This study was conducted under a data use agreement between the NIDDK and W.C.W. The manuscript was reviewed by an NIDDK officer and approved for publication. The data reported here have been supplied by the United States Renal Data System and by DaVita, Inc. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation.
REFERENCES
- 1.Cronin RE, Reilly RF. Unfractionated heparin for hemodialysis: still the best option. Semin Dial. 2010;23:510–515. doi: 10.1111/j.1525-139X.2010.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol. 2009;20:705–711. doi: 10.1681/ASN.2007111207. [DOI] [PubMed] [Google Scholar]
- 3.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. J Am Soc Nephrol. 2009;20:872–881. doi: 10.1681/ASN.2008080824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phelan PJ, O'Kelly P, Holian J, et al. Warfarin use in hemodialysis patients: what is the risk? Clin Nephrol. 2011;75:204–211. doi: 10.5414/cn106481. [DOI] [PubMed] [Google Scholar]
- 5.Pinson CW, Schuman ES, Gross GF, Schuman TA, Hayes JF. Surgery in long-term dialysis patients. Experience with more than 300 cases. Am J Surg. 1986;151:567–571. doi: 10.1016/0002-9610(86)90548-9. [DOI] [PubMed] [Google Scholar]
- 6.Holden RM, Harman GJ, Wang M, Holland D, Day AG. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:105–110. doi: 10.2215/CJN.01810407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caruana RJ, Raja RM, Bush JV, Kramer MS, Goldstein SJ. Heparin free dialysis: comparative data and results in high risk patients. Kidney Int. 1987;31:1351–1355. doi: 10.1038/ki.1987.149. [DOI] [PubMed] [Google Scholar]
- 8.Keller F, Seemann J, Preuschof L, Offermann G. Risk factors of system clotting in heparin-free haemodialysis. Nephrol Dial Transplant. 1990;5:802–807. doi: 10.1093/ndt/5.9.802. [DOI] [PubMed] [Google Scholar]
- 9.Casati S, Moia M, Graziani G, et al. Hemodialysis without anticoagulants: efficiency and hemostatic aspects. Clin Nephrol. 1984;21:102–105. [PubMed] [Google Scholar]
- 10.Preuschof L, Keller F, Seemann J, Offermann G. Heparin-free hemodialysis with prophylactic change of dialyser and blood lines. Int J Artif Organs. 1988;11:255–258. [PubMed] [Google Scholar]
- 11.Sanders PW, Taylor H, Curtis JJ. Hemodialysis without anticoagulation. Am J Kidney Dis. 1985;5:32–35. doi: 10.1016/s0272-6386(85)80132-3. [DOI] [PubMed] [Google Scholar]
- 12.Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–2233. doi: 10.1681/ASN.2009030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662–2668. doi: 10.2215/CJN.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77:1098–1106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 15.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Yang JY, Lee TC, Montez-Rath ME, et al. Trends in acute nonvariceal upper gastrointestinal bleeding in dialysis patients. J Am Soc Nephrol. 2012;23:495–506. doi: 10.1681/ASN.2011070658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrade SE, Harrold LR, Tjia J, et al. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):100–128. doi: 10.1002/pds.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):154–162. doi: 10.1002/pds.2341. [DOI] [PubMed] [Google Scholar]
- 19.Rural Urban Commuting Area Codes Data. 2012 http://depts.washington.edu/uwruca/ruca-data.php. (5 January 2012, date last accessed) [Google Scholar]
- 20.Census Bureau Regions and Divisions with State FIPS Codes. 2012 http://www.census.gov/geo/www/reg_div.txt. (5 January 2012, date last accessed) [Google Scholar]
- 21.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 23.Suhocki PV, Conlon PJ, Jr, Knelson MH, Harland R, Schwab SJ. Silastic cuffed catheters for hemodialysis vascular access: thrombolytic and mechanical correction of malfunction. Am J Kidney Dis. 1996;28:379–386. doi: 10.1016/s0272-6386(96)90495-3. [DOI] [PubMed] [Google Scholar]
- 24.Lund GB, Trerotola SO, Scheel PF, Jr., et al. Outcome of tunneled hemodialysis catheters placed by radiologists. Radiology. 1996;198:467–472. doi: 10.1148/radiology.198.2.8596851. [DOI] [PubMed] [Google Scholar]
- 25.Allon M. Current management of vascular access. Clin J Am Soc Nephrol. 2007;2:786–800. doi: 10.2215/CJN.00860207. [DOI] [PubMed] [Google Scholar]
- 26.Mokrzycki MH, Lok CE. Traditional and non-traditional strategies to optimize catheter function: go with more flow. Kidney Int. 2010;78:1218–1231. doi: 10.1038/ki.2010.332. [DOI] [PubMed] [Google Scholar]
- 27.Hirth RA, Turenne MN, Woods JD, et al. Predictors of type of vascular access in hemodialysis patients. JAMA. 1996;276:1303–1308. [PubMed] [Google Scholar]
- 28.Reddan D, Klassen P, Frankenfield DL, et al. National profile of practice patterns for hemodialysis vascular access in the United States. J Am Soc Nephrol. 2002;13:2117–2124. doi: 10.1097/01.asn.0000022422.79790.a8. [DOI] [PubMed] [Google Scholar]
- 29.Stack AG. Determinants of modality selection among incident US dialysis patients: results from a national study. J Am Soc Nephrol. 2002;13:1279–1287. doi: 10.1681/ASN.V1351279. [DOI] [PubMed] [Google Scholar]
- 30.Reddan DN, Frankenfield DL, Klassen PS, et al. Regional variability in anaemia management and haemoglobin in the US. Nephrol Dial Transplant. 2003;18:147–152. doi: 10.1093/ndt/18.1.147. [DOI] [PubMed] [Google Scholar]
- 31.Shen JI, Winkelmayer WC. Use and safety of unfractionated heparin for anticoagulation during maintenance hemodialysis. Am J Kidney Dis. 2012;60:473–486. doi: 10.1053/j.ajkd.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasse H, Gillen DL, Ball AM, et al. Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int. 2003;64:1455–1461. doi: 10.1046/j.1523-1755.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 33.Kawamura M, Fijimoto S, Hisanaga S, Yamamoto Y, Eto T. Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis. 1998;31:991–996. doi: 10.1053/ajkd.1998.v31.pm9631844. [DOI] [PubMed] [Google Scholar]
- 34.Bhasin HK, Dana CL. Spontaneous retroperitoneal hemorrhage in chronically hemodialyzed patients. Nephron. 1978;22:322–327. doi: 10.1159/000181470. [DOI] [PubMed] [Google Scholar]
- 35.Vanichayakornkul S, Cioffi RF, Harper E, O'Connell JM, Shalhoub RJ. Spontaneous retroperitoneal hematoma. A complication of hemodialysis. JAMA. 1974;230:1164–1165. [PubMed] [Google Scholar]
- 36.De Santo NG, Capodicasa G, Perna N, De Pascale C, Giordano C. Haematoma of rectus abdominis associated with dialysis. Br Med J. 1972;3:281–282. doi: 10.1136/bmj.3.5821.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milutinovich J, Follette WC, Scribner BH. Spontaneous retroperitoneal bleeding in patients on chronic hemodialysis. Ann Intern Med. 1977;86:189–192. doi: 10.7326/0003-4819-86-2-189. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi H, Kurata Y, Imanaga Y, Goya K, Oshima K. Vitrectomy for diabetic retinopathy in patients undergoing hemodialysis for associated end-stage renal failure. Retina. 1998;18:156–159. doi: 10.1097/00006982-199818020-00010. [DOI] [PubMed] [Google Scholar]
- 39.Slusher MM, Hamilton RW. Letter: Spontaneous hyphema during hemodialysis. N Engl J Med. 1975;293:561. [PubMed] [Google Scholar]
- 40.Galen MA, Steinberg SM, Lowrie EG, Lazarus JM, Hampers CL, Merrill JP. Hemorrhagic pleural effusion in patients undergoing chronic hemodialysis. Ann Intern Med. 1975;82:359–361. doi: 10.7326/0003-4819-82-3-359. [DOI] [PubMed] [Google Scholar]
- 41.Alfrey AC, Goss JE, Ogden DA, Vogel JH, Holmes JH. Uremic hemopericardium. Am J Med. 1968;45:391–400. doi: 10.1016/0002-9343(68)90073-9. [DOI] [PubMed] [Google Scholar]
- 42.Slinin Y, Guo H, Li S, et al. Association of provider–patient visit frequency and patient outcomes on hemodialysis. J Am Soc Nephrol. 2012;23:1560–1567. doi: 10.1681/ASN.2012010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.