Abstract
Silicone lymphadenopathy is a recognized complication of silicone gel implant rupture; the ipsilateral axillary lymph nodes are most commonly involved. We report imaging findings on a range of different imaging modalities and biopsy results in a case of biopsy-proven silicone lymphadenitis involving contralateral intramammary and axillary lymph nodes in a patient with an intact standard dual-lumen breast implant in the opposite reconstructed breast. This case demonstrates that in a patient with disrupted lymph drainage due to prior mastectomy and axillary node dissection for breast cancer treatment, silicone particles can migrate in a retrograde fashion via the ipsilateral internal mammary lymph nodes and reach not only the contralateral axilla but also the outer quadrants of the contralateral breast, even in the presence of an intact breast implant.
Keywords: Breast, Implant, Silicone, Lymphadenitis, Mastectomy
CASE REPORT
A 60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant and no prior right breast surgery or implant placement presented to our center for mammographic and sonographic evaluation following reported abnormal findings on a combined tumor and breast implant protocol breast Magnetic Resonance (MR) exam at an outside institution and a whole-body Positron Emission Tomography-Computerized Tomography (PET-CT) exam at our institution.
Imaging Findings
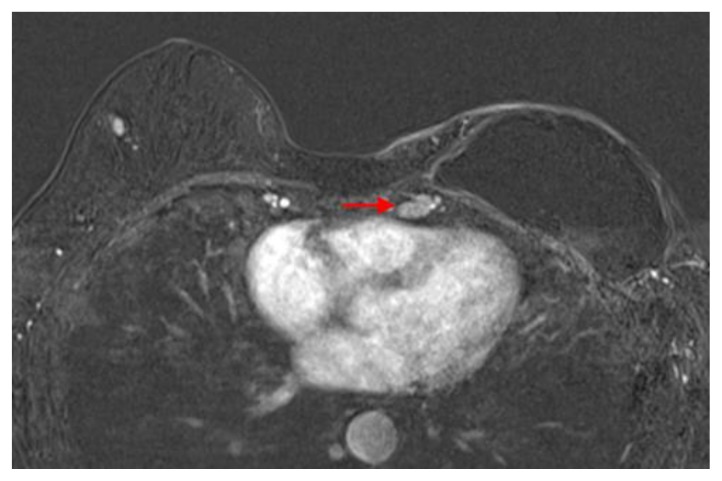
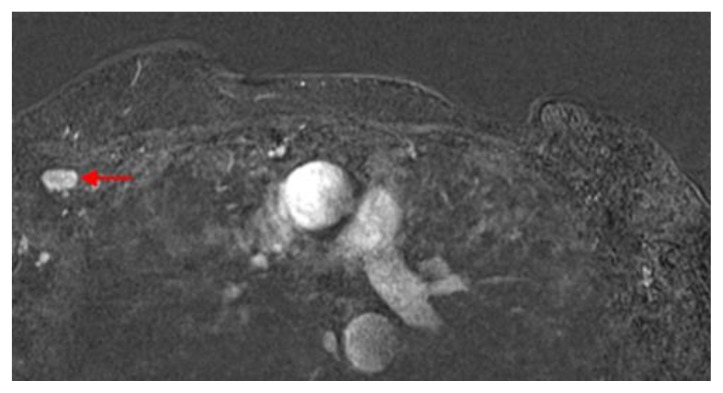
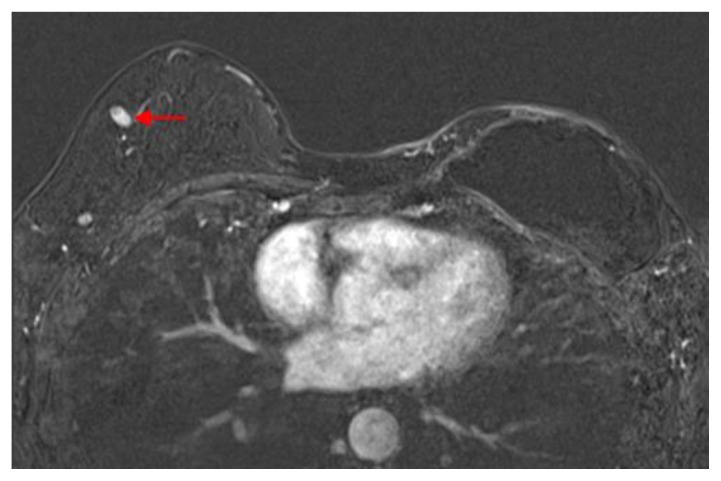
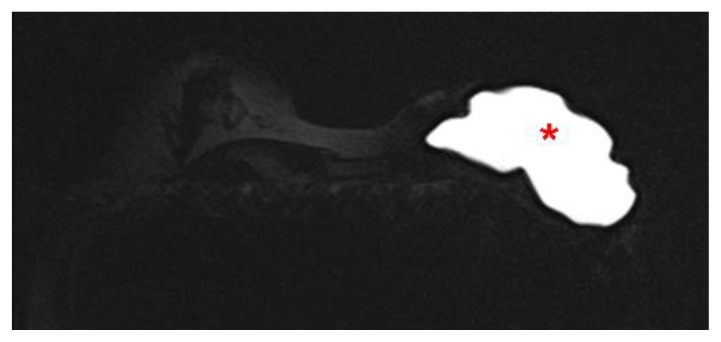
Contrast enhanced subtracted breast MR images demonstrated abnormal appearing left internal mammary lymph node measuring 1.6 × 0.9 × 1.4 cm, abnormal appearing right axillary lymph node measuring 1.5 × 0.9 × 1.3 cm and abnormal appearing intramammary right breast lymph node measuring 1.0 × 0.8× 1.4 cm (Figs 1, 2 and 3). Dedicated MR breast implant sequences without contrast demonstrated an intact standard dual lumen left breast implant (outer saline/inner silicone) (Fig 4 and 5).
Figure 1.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy axial T1 weighted MR 1 minute post contrast subtracted image (TR 3.69 TE 1.35; 1.5T Siemens MAGNETOM Sonata MRI scanner using breast phased array coil; matrix 320×320; 18 cc of Prohance) demonstrates enlarged lymph node with cortical thickening in the left internal mammary chain measuring 1.6 × 0.9 × 1.4 cm.
Figure 2.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy axial T1 weighted MR 1 minute post contrast subtracted image (TR 3.69 TE 1.35; 1.5T Siemens MAGNETOM Sonata MRI scanner using breast phased array coil; matrix 320×320; 18 cc of Prohance) demonstrates enlarged lymph node with cortical thickening in the right axilla measuring 1.5 × 0.9 × 1.3 cm.
Figure 3.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy axial T1 weighted MR 1 minute post contrast subtracted image (TR 3.69 TE 1.35; 1.5T Siemens MAGNETOM Sonata MRI scanner using breast phased array coil; matrix 320×320; 18 cc of Prohance) demonstrates enlarged lymph node with cortical thickening in the right breast upper outer quadrant measuring 1.0 × 0.8 × 1.4 cm.
Figure 4.
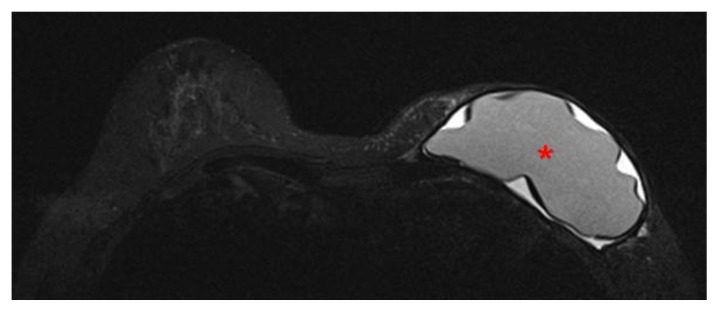
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy axial STIR MR image (TR 4000 TE 72; 1.5T Siemens MAGNETOM Sonata MRI scanner using breast phased array coil; matrix 384×384) demonstrates an intact standard dual lumen (inner silicone, outer saline) breast implant in a reconstructed left breast.
Figure 5.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy axial water-suppressed MR image (TR 4600 TE 61; 1.5T Siemens MAGNETOM Sonata MRI scanner using breast phased array coil; matrix 320×314) demonstrates an intact inner silicone lumen in an standard dual lumen (inner silicone, outer saline) breast implant in a reconstructed left breast.
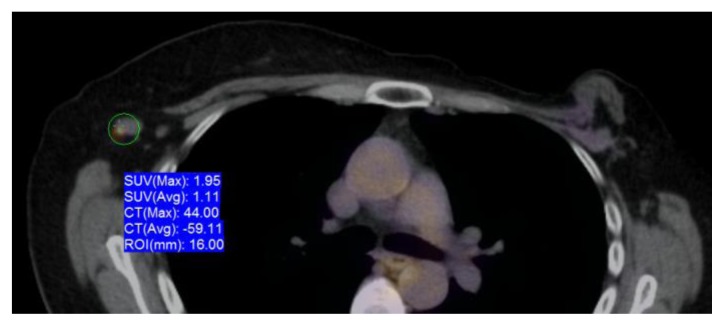
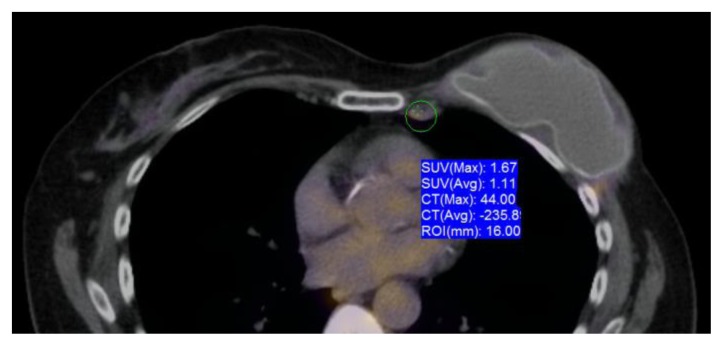
PET-CT exam demonstrated a morphologically abnormal right axillary lymph node measuring 1.4 × 0.9 cm, without a central fatty hilum and with increased metabolic activity with maximum standardized uptake value (SUVmax) of 1.95 as well as a left internal mammary lymph node measuring 1.6 × 1.0 cm with increased metabolic activity with a SUVmax of 1.67 (Figs 6 and 7).
Figure 6.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy axial fusion image from an FDG PET/CT exam performed 90 minutes after the injection of 13.8 mCi of FDG demonstrates a morphologically abnormal right axillary lymph node measuring 1.4 × 0.9 cm, without a central fatty hilum with increased metabolic activity with SUVmax of 1.95.
Figure 7.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy axial fusion images from a pre biopsy FDG PET/CT exam performed 90 minutes after the injection of 13.8 mCi of FDG demonstrates a morphologically abnormal left internal mammary lymph node measuring 1.6×1.0 cm with increased metabolic activity with SUVmax of 1.67.
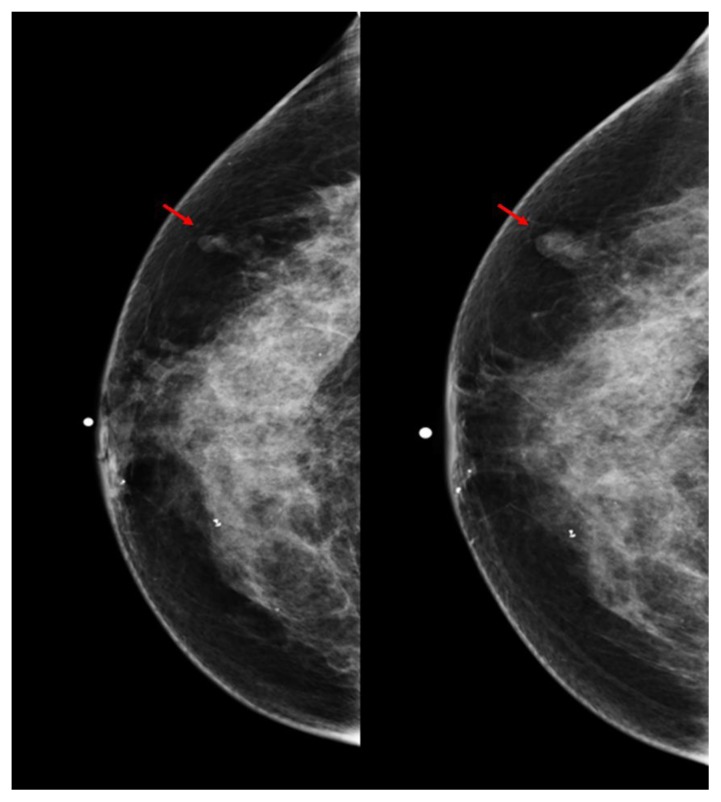
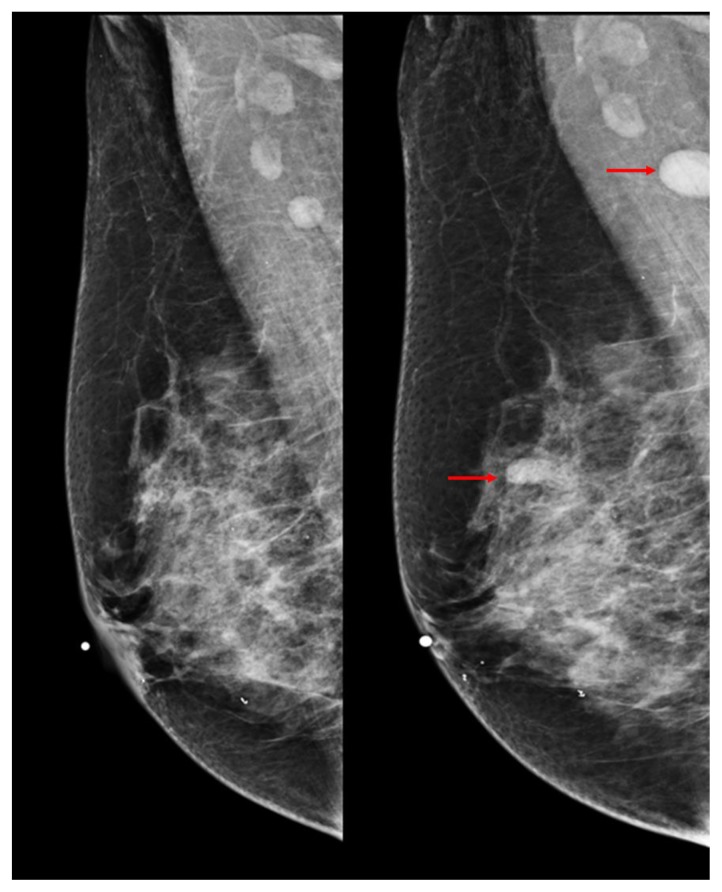
Full-field digital diagnostic craniocaudal (CC) and mediolateral oblique (MLO) mammographic images of the right breast demonstrated interval increase in size and radiodensity of previously noted normal-appearing lymph nodes in the right axilla (measuring 0.8 × 0.9 cm and 1.1 × 1.1 cm on 9/2008 and 10/2012 respectively) and in the right breast upper outer quadrant (measuring 0.6 × 0.4 cm and 1.1 × 0.6 cm on 9/2008 and 10/2012 respectively) (Figs 8 and 9).
Figure 8.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy right breast craniocaudal mammograms dated 9/2008 and 10/2012 (kVp: 30; mAs: 72; and kVp: 29; mAs: 94, respectively) demonstrate interval increased size and radiodensity of an initially normal appearing intramammary lymph node in the right upper outer quadrant measuring 0.6 × 0.4 cm which on follow-up study measured 1.1 × 0.6 cm.
Figure 9.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy right breast mediolateral mammograms dated 9/2008 and 10/2012 (kV: 29; mAs: 73, and kV: 29; mAs: 74, respectively) demonstrate interval increased size and radiodensity of previously noted normal-appearing right axillary lymph node initially measured at 0.8 × 0.9cm which on follow-up study measured 1.1 × 1.1cm. Interval increased size and radiodensity of an initially normal appearing intramammary lymph node in the right upper outer quadrant measuring 0.6 × 0.4 cm which on follow-up study measured 1.1 × 0.6 cm is also demonstrated.
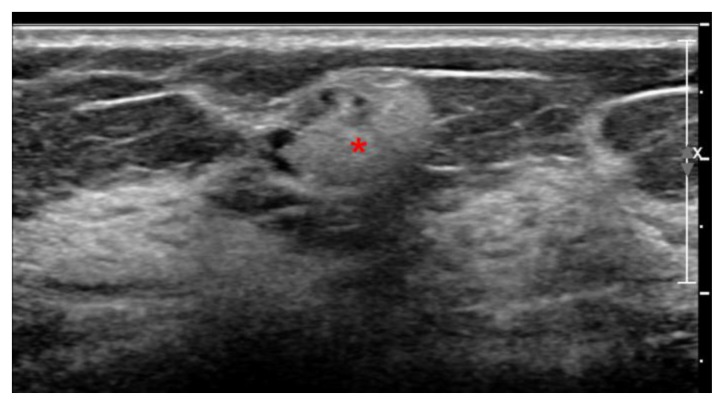
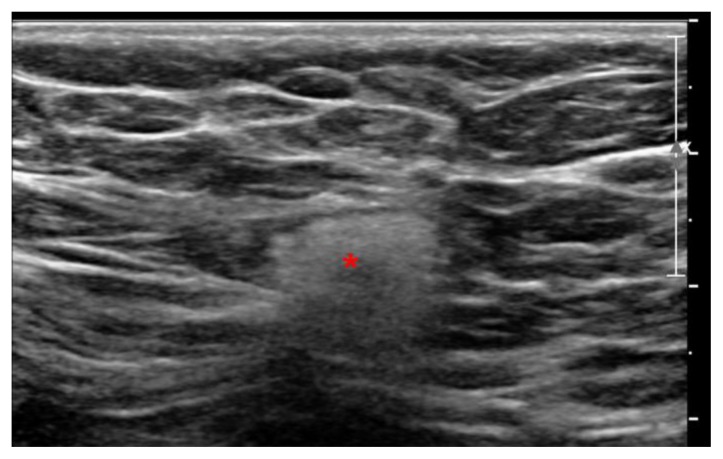
Ultrasound images demonstrated markedly hyperechoic lymph nodes with dirty shadowing (snowstorm appearance) in the right axilla measuring 1.5 × 1.5 × 1 cm, the right breast upper outer quadrant measuring 1.3 × 0.9 × 0.8 cm (Figs 10 and 11).
Figure 10.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy gray scale ultrasound image of the right breast using a high frequency linear probe demonstrate markedly hyperechoic intramammary lymph node with snow storm artifact measuring 1.3 × 0.9 × 0.8 cm.
Figure 11.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Pre biopsy gray scale ultrasound image of the right axilla using a high frequency linear probe demonstrate markedly hyperechoic axillary lymph node with snow storm artifact measuring 1.5 × 1.5× 1.0 cm.
Management and Follow up
Due to the history of breast cancer, ultrasound-guided core needle biopsies of the abnormal-appearing right axillary and right breast intramammary lymph nodes were performed. Three specimens were obtained from each lymph node with a 14-gauge Achieve biopsy needle (Cardinal Health, Dublin, Ohio). Because of the technical difficulty and higher risk of complication, the abnormal internal mammary chain lymph node was not biopsied. This lymph node had similar imaging appearance to the biopsied lymph nodes, so it was considered to represent the same pathologic process. Both biopsies yielded Silicone Lymphadenitis (Fig 6). Biopsy findings were considered concordant with the imaging features and a short term follow up with right breast mammogram at 6 months was recommended.
DISCUSSION
Etiology
Silicone particles can migrate through tissues following either overt breast implant rupture or slow gel bleed through an apparently intact outer implant shell [1–5]. Risk of breast implant rupture and leakage increases with increasing age of the implant, and depends on the site of implantation (most likely if subglandular as opposed to retropectoral), the presence of local tissue contractures and type of implant used [1,6].
Once outside the confines of the implant silicone particles may be transported to regional lymph nodes by macrophages and generate a granulomatous reaction which may present as lymphadenopathy with the ipsilateral axillary lymph nodes being most commonly involved [2,4]. Involvement of ipsilateral intramammary, internal mammary, supraclavicular as well as of contralateral internal mammary and axillary lymph nodes has also been reported [3–5].
Lymphadenopathy in patients with history of breast cancer raises concern for recurrence and warrants prompt evaluation to avoid delay in proper diagnosis and treatment. There have been reports of both silicone granulomata and breast cancer metastases coexisting in the same lymph node [8,9].
Double lumen breast implant
Standard double lumen breast implant as the one present in our case, have an inner silicone gel chamber and an outer saline chamber intended to provide a “second layer of protection” against implant breakage and are less likely to rupture than single lumen implants [1,10]. This type of breast implant is designed to prevent massive deflation and asymmetry in case of rupture of one chamber and to allow for adjustments of the saline chamber at the time of surgery for better symmetry.
Lymphatic drainage of breast
In the breast, more than 75% of the lymph drainage, particularly from the outer quadrants, drains to the ipsilateral axillary lymph nodes. The remainder drains to either the internal mammary lymph nodes, the opposite breast inner quadrants or to the inferior phrenic nodes (particularly from the lower quadrants) [7].
It has been postulated that regional spread of breast cancer in patients whose normal lymphatic pathways may have been disrupted or obstructed by scarring from surgery, including axillary lymph node dissection can occur in a retrograde direction through alternate lymphatic pathways [11,12].
Clinical presentation and Imaging features
Patients with silicone lymphadenitis can be asymptomatic. At the time imaging studies are obtained, a history of silicone breast implant may be all the history that is provided. Clinically, a palpable lump or focal pain can also be the reason for further evaluation.
Mammographic findings are non-specific with detection of normal or enlarged lymph nodes, which could show increased density later on (as it did in our case). Suspicious mammographic findings prompt further evaluation with ultrasound.
Silicone lymphadenopathy involves accumulation of silicone gel first in the medullary sinuses (unlike metastasis, which primarily involves the lymph node cortex). On ultrasound this results in subtle, ill-defined, incoherent posterior acoustic shadowing arising from the lymph node mediastinum. As amount of silicone gel increases, the hilum becomes more hyperechoic, and shadowing assumes a more classic “snowstorm” appearance. Eventually, even the cortex becomes hyperechoic, giving an overall snowstorm appearance of the lymph node [13].
Most implant ruptures are not clinically apparent nor are they readily visible on routine mammographic/sonographic imaging. MRI is the most accurate imaging modality to evaluate the integrity of breast silicone implants. However, lymph node morphology is better evaluated by ultrasound. Current FDA recommendations for silent implant rupture screening are breast MRI implant protocol three years following implant placement and every two years thereafter.
Treatment and Prognosis
Confirmation of the silicone lymphadenopathy by biopsy can be done and it is necessary to exclude coexistent malignancy specifically in a patient with history of breast or other malignancies. Once malignancy is excluded, conservative approach with follow up on imaging and/or excision if intramammary lymph nodes are present is usually the course of treatment with excellent prognosis.
Differential Diagnosis
Differential diagnosis includes metastatic, infectious and inflammatory lymphadenopathy and recurrent cancer. Except for very early cases of silicone migration to involved nodes, silicone lymphadenitis is usually distinguishable from the other entities due to presence of silicone particles in the involved node leading to dense particles on mammography and snowstorm appearance or dirty shadowing on ultrasound. Recurrent and metastatic cancer needs to be excluded by biopsy in patients with personal history of breast and other non-breast malignancies when typical features of silicone lymphadenitis are absent.
Histological features
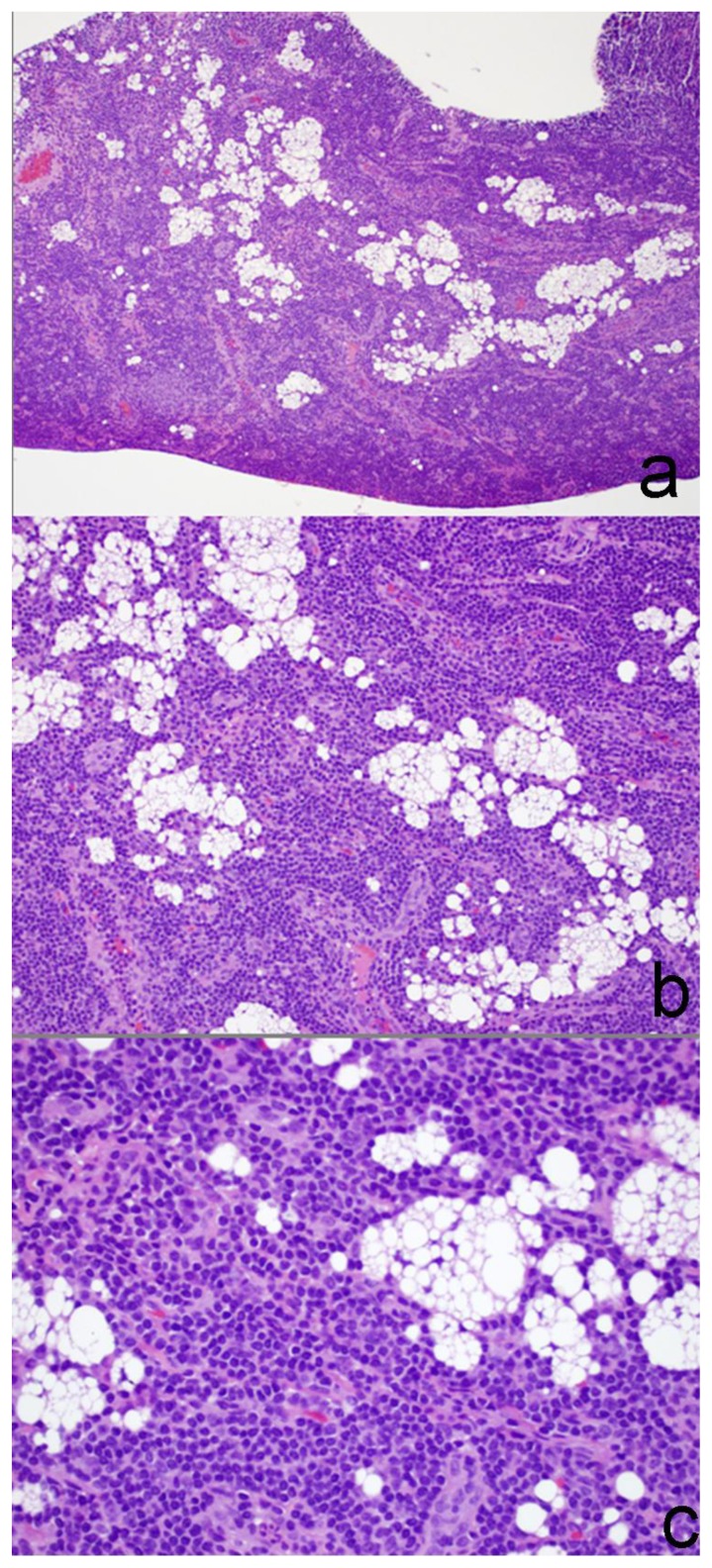
Lymph nodes affected by silicone lymphadenitis generally do not manifest specific gross features on pathology. The histologic appearance can vary widely, ranging from uninvolved to global infiltration. Histologic features include diffuse follicular hyperplasia with interspersed histiocytes with clear, vacuolated cytoplasm. Foreign-body type giant cells, some containing refractile material, may accumulate in areas where clusters of clear cells have formed empty vacuoles. Silicone gel from mammary prostheses typically produces fine vacuolated deposits. As there is no histochemical or immunohistochemical procedure that can stain silicone, a definitive identification of silicone in lymph nodes and other tissues can be confirmed by electron microprobe analysis using transmission or scanning electron microscopy [14].
TEACHING POINT
Even in the presence of an intact breast implant, patients status-post breast reconstruction after surgical treatment for breast cancer are at increased risk of contralateral axillary and intrammamary silicone lymphadenitis due to the disrupted breast lymph drainage. Even in the presence of pathognomonic features of silicone lymphadenitis as best demonstrated on ultrasound, image guided biopsy may be considered to rule out malignancy, due to the history of breast cancer.
Figure 12.
60 year old female status post left breast modified radical mastectomy and left axillary lymph node dissection followed by left breast reconstruction with a standard dual-lumen breast implant, with biopsy-proven silicone lymphadenitis of contralateral (right) axillary and intramammary lymph nodes. Haematoxilin and eosin stained microphotograph of sample from a core needle biopsy of the right breast intramammary lymph node at ×10 power (a), at ×20 power (b) and at ×40 power (c) demonstrates diffuse follicular hyperplasia with interspersed histiocytes with clear, vacuolated cytoplasm, foreign-body type giant cells and fine vacuolated deposits.
Table 1.
Summary table for Silicone Lymphadenitis due to breast silicone implant rupture
| Etiology | Overt silicone gel breast implant rupture or slow gel bleed through an apparent intact outer implant shell. |
| Incidence | No study has directly examined the incidence silicone lymphadenitis due to breast silicone implant rupture. The incidence of MRI-diagnosed breast silicone ruptures has been calculated at 4.4 ruptures/100 implants per year. |
| Gender ratio | Female overwhelmingly more common as breast implants in male subjects are rarely used. |
| Age predilection | The incidence rate of breast silicone implant rupture (and thus that of silicone lymphadenitis) increases with implant age and is more common after 10 years of implant placement. |
| Risk factors | Presence of silicone gel breast implant, increasing age of implant, site of implant (more likely if subglandular as opposed to retropectoral), presence of local contractures, type of implant, disrupted lymphatic drainage due to prior mastectomy and axillary node dissection. |
| Treatment | None. Excision may be considered for intramammary lymph nodes with silicone lymphadenitis |
| Prognosis | Excellent, if associated malignancy is excluded. |
| Imaging findings | Mammography: Enlarged and dense lymph nodes indistinguishable from metastatic nodes. Ultrasound: Enlarged hyperechoic lymph nodes with snow storm artifact. MRI: Enlarge lymph nodes, predominantly low signal intensity on T1W and high signal intensity on T2W images with enhancement on post contrast study. |
Table 2.
Differential diagnosis for Silicone Lymphadenitis due to breast silicone implant rupture
| Differential Diagnosis | Mammography | Ultrasound | MRI | PET-CT |
|---|---|---|---|---|
| Silicone lymphadenitis | Dense lymph nodes +/− containing calcifications/hyperdense material/free silicone | Central echogenicity with classic ‘dirty shadowing’ | T1 hypo, T2 Hyper, drop in signal on silicone suppressed sequences | Variable, can be hypermetabolic |
| Metastatic lymphadenopathy: breast, lung, melanoma, thyroid, ovary, GI tract, lymphoma/leukemia | Dense, round/irregular/ill-defined lymph node, absence of normal central hilar fat, abnormal calcification. | Eccentric enlargement with focal thickening of cortex of the lymph node with obliteration of fatty hilum. | Low T1/high T2 signal intensity with enhancement. | Increased metabolic activity. |
| Infectious disease: local breast infection/abscess, HIV, Cat Scratch disease | Well defined enlarged lymph nodes | Symmetric enlargement with diffuse thickening of lymph node cortex. | Low T1/high T2 signal intensity with enhancement | Increased metabolic activity. |
| Inflammatory disease: sarcoidosis, rheumatoid arthritis, systemic lupus erythematosus | Well defined enlarged lymph nodes | Symmetric enlargement with diffuse thickening of lymph node cortex. | Low T1/high T2 signal intensity with enhancement. | May show increased metabolic activity. |
ABBREVIATIONS
- MR
Magnetic Resonance
- PET-CT
Positron Emission Tomography-Computerized Tomography
- SUVmax
Maximum standardized uptake value
REFERENCES
- 1.Steinbach BG, Hardt NS, Abbitt PL, Lanier L, Caffee HH. Breast implants, common complications, and concurrent breast disease. Radiographics. 1993 Jan;13(1):95–118. doi: 10.1148/radiographics.13.1.8426939. [DOI] [PubMed] [Google Scholar]
- 2.Adams ST, Cox J, Rao GS. Axillary silicone lymphadenopathy presenting with a lump and altered sensation in the breast: a case report. J Med Case Rep. 2009 Mar 10;3:6442. doi: 10.1186/1752-1947-3-6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman GJ, Sakr RA, Inguenault C, Sarfati I, Nos C, Clough KB. Silicone migration to the contralateral axillary lymph nodes and breast after highly cohesive silicone gel implant failure: a case report. Cases J. 2009 Mar 10;2:6420. doi: 10.1186/1757-1626-2-6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatima H, Weiss CG, Mohammad I. Silicone Lymphadenopathy in a 48-Year Old Female with a History of Breast Cancer- A Case report with Cytologic Findings. [Accessed February 3, 2013];Int J Pathol. 2007 5(2):86–8. Available at: http://www.pakmedinet.com/12595. [Google Scholar]
- 5.Kao CC, Rand RP, Holt CA, Pierce RH, Timmons JH, Wood DE. Internal mammary silicone lymphadenopathy mimicking recurrent breast cancer. Plast Reconstr Surg. 1997 Jan;99(1):225–9. doi: 10.1097/00006534-199701000-00034. [DOI] [PubMed] [Google Scholar]
- 6.Middleton MS, McNamara MP., Jr Breast implant classification with MR imaging correlation: (CME available on RSNA link) Radiographics. 2000 May;20(3):E1. doi: 10.1148/radiographics.20.3.g00mae11. [DOI] [PubMed] [Google Scholar]
- 7.Tanis PJ, Nieweg OE, Valdés Olmos RA, Kroon BB. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg. 2001 Mar;192(3):399–409. doi: 10.1016/s1072-7515(00)00776-6. Review. [DOI] [PubMed] [Google Scholar]
- 8.Tabatowski K, Elson CE, Johnston WW. Silicone lymphadenopathy in a patient with a mammary prosthesis. Fine needle aspiration cytology, histology and analytical electron microscopy. Acta Cytol. 1990 Jan-Feb;34(1):10–4. PubMed. [PubMed] [Google Scholar]
- 9.Santos-Briz A, Jr, López-Ríos F, Santos-Briz A, De Agustín PP. Granulomatous reaction to silicone in axillary lymph nodes. A case report with cytologic findings. Acta Cytol. 1999 Nov-Dec;43(6):1163–5. doi: 10.1159/000331373. PubMed. [DOI] [PubMed] [Google Scholar]
- 10.Hölmich LR, Friis S, Fryzek JP, Vejborg IM, Conrad C, Sletting S, Kjøller K, McLaughlin JK, Olsen JH. Incidence of silicone breast implant rupture. Arch Surg. 2003 Jul;138(7):801–6. doi: 10.1001/archsurg.138.7.801. [DOI] [PubMed] [Google Scholar]
- 11.Eubank WB, Mankoff DA, Vesselle HJ, Eary JF, Schubert EK, Dunnwald LK, Lindsley SK, Gralow JR, Austin-Seymour MM, Ellis GK, Livingston RB. Detection of locoregional and distant recurrences in breast cancer patients by using FDG PET. Radiographics. 2002 Jan-Feb;22(1):5–17. doi: 10.1148/radiographics.22.1.g02ja055. Review PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Wellner R, Dave J, Kim U, Menes TS. Altered lymphatic drainage after breast-conserving surgery and axillary node dissection: local recurrence with contralateral intramammary nodal metastases. Clin Breast Cancer. 2007 Feb;7(6):486–8. doi: 10.3816/CBC.2007.n.006. [DOI] [PubMed] [Google Scholar]
- 13.Stavros TA. Breast Ultrasound. 1st ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 834–876. [Google Scholar]
- 14.Rosen PP. Rosen’s Breast Pathology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 922–923. [Google Scholar]