Abstract
We present a case of a postmenopausal woman diagnosed with an ovarian mass containing thyroid follicles and foci of papillary thyroid carcinoma during pathological examination. This patient referred having had a metachronous thyroid malignancy 10 years before. The differential diagnosis between a thyroid malignancy arising from a struma ovarii and a metastatic ovarian tumor originating from thyroid-cancer is challenging. Struma ovarii should be considered when thyroid components are the predominant element or when thyroid malignant tissue is identified within an ovarian lesion. Thyroid carcinoma arising from a struma ovarii is reported to occur in a minority of cases. Of these, papillary carcinoma is the most frequent subtype encountered. Regarding primary thyroid carcinomas, papillary carcinomas have a lower metastatic potential when compared to follicular carcinomas, and most of the metastases occur in the cervical lymph nodes. Ovarian metastases are exceedingly rare and generally associated with widespread disease. However, they must be considered in the presence of previous history of malignant thyroid carcinoma. The authors review the main clinical, imaging and therapeutic aspects of both these entities and present the most likely diagnosis.
Keywords: Urogenital System, Ovarian Neoplasms, Papillary thyroid carcinoma, Ovarian metastasis, Struma ovarii, Thyroid neoplasms
CASE REPORT
A 78-year-old woman complaining of pelvic pain and significant weight loss was referred for gynecological examination.
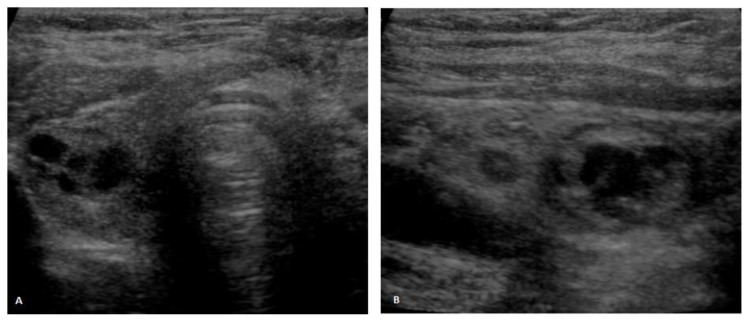
Her medical history was positive for multinodular goiter. A partial left thyroidectomy had been performed ten years before, after a tumor measuring 8×5×4,5cm was diagnosed. Although the latter was initially thought to be a follicular adenoma, a subsequent reassessment was performed at our institution and the microscopic findings were considered to be a follicular variant of a papillary thyroid carcinoma. No therapy was further purposed and the thyroid ultrasounds performed periodically thereafter revealed no significant variation in size of the multiple nodules detected. The latter measured up to 1.5cm in greatest axis and presented either a mixed solid and cystic and a predominantly cystic nature (fig. 1). There was no history of either benign or malignant thyroid disease in the patient’s family.
Figure 1.
Thyroid ultrasound. 71-year-old female patient with an ovarian lesion containing thyroid follicles and foci of papillary carcinoma, most probably representing a papillary carcinoma arising in a struma ovarii. Ultrasound scans in axial (1A) and sagittal (1B) planes depict multiple nodules in the right thyroid lobe, measuring up to 1.5cm in greatest axis, some of them being mixed solid and cystic and the others being predominantly cystic. None of the nodules exhibited suggestive features of malignancy [GE Voluson V730, linear transducer, 37 Hz].
During physical examination, a large mass was found on the right lower quadrant of the abdomen.
The routine laboratory data, the serum level of CA-125 and the levels of circulating thyroid hormones - thyroid stimulating hormone (TSH), free T4 and thyroglobulin, were within the normal range.
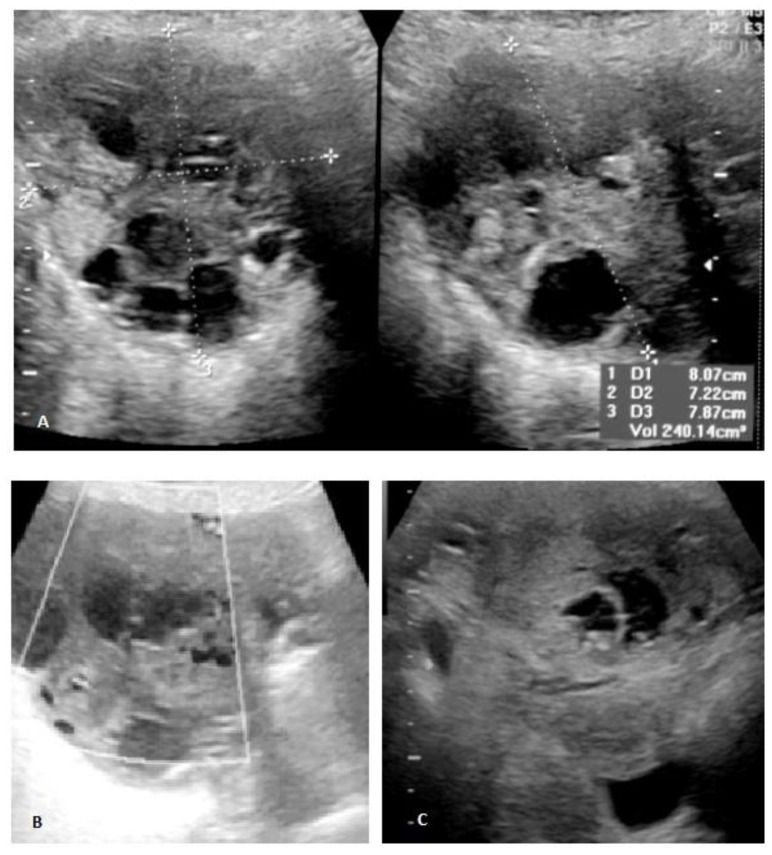
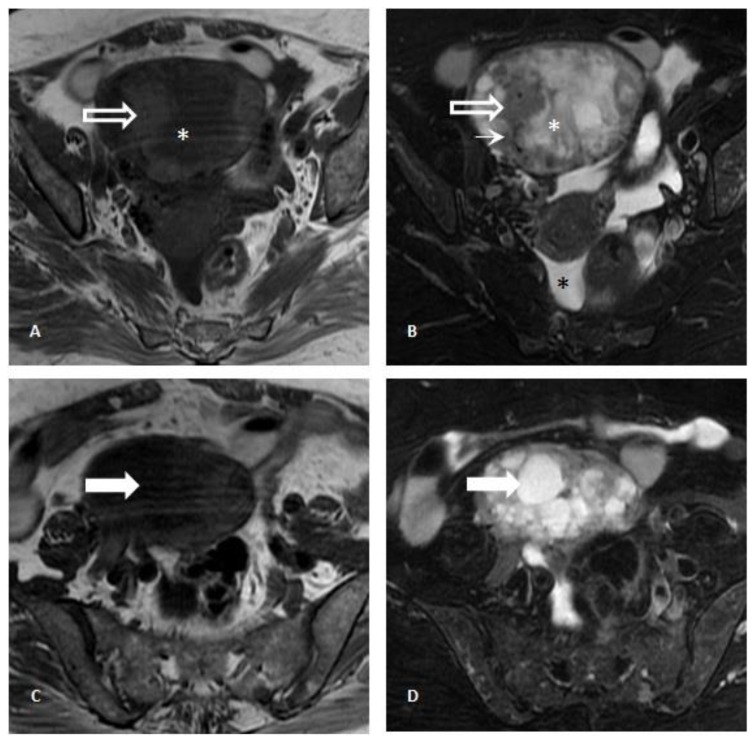
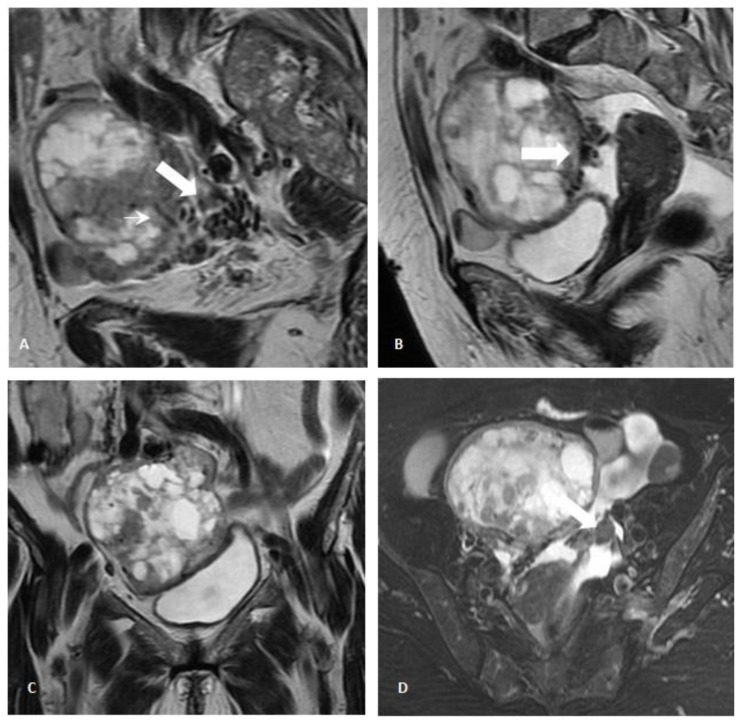
Pelvic transabdominal ultrasound revealed a partially solid and cystic mass in the right adnexal area, measuring 8×7,9×7,2cm (fig. 2A). The identification of both thick septations and solid well-vascularized components raised the suspicion for an ovarian malignancy (figs. 2B and 2C). A pelvic MR imaging was subsequently performed (fig. 3) and revealed a predominantly multiloculated mass presenting thickened septa and a variable signal intensity between locules (“stained glass appearance”). Some of these locules showed low signal intensity on both T1- and T2-weighted images (figs. 3A and 3B; white asterisk), while others exhibited low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (figs. 3C and 3D; white arrow). The lesion had also solid components (figs. 3A and B; white-bordered arrow), which presented intermediate to low signal on both T2- and T1-weighted images. The presence of signal voids within the latter (figs. 3A and B; thin white arrow) and the visualization of numerous perilesional vessels nourishing the tumor reflected its hypervascular nature (figs. 4A and 4B; white arrow). Nonetheless, further evaluation with gadolinium administrated was not possible upon patient refusal. No fatty components within the lesion were detected on the fat-saturation T2-weighted images (figs. 3B and 3D). A small amount of ascites was additionally detected in the pouch of Douglas / cul-de-sac (figs. 3B and 4B; black asterisk).
Figure 2.
Pelvic transabdominal ultrasound. 71-year-old female patient with an ovarian lesion containing thyroid follicles and foci of papillary carcinoma, most probably representing a papillary carcinoma arising in a struma ovarii. A heterogeneous complex-appearing right adnexal mass, containing solid and cystic areas, was detected (2A). The presence of several thick septations and solid areas within the tumor (2A and 2B), as well as the identification of ascites in the cul de sac (2C) raised the suspicion for an ovarian malignant neoplasm. Color Doppler ultrasound revealed flow in the septa and in the solid areas (2B) [GE Voluson V730, curved transducer, 10–15 Hz].
Figure 3.
Pelvic Magnetic Resonance Imaging. 71-year-old female patient with an ovarian lesion containing thyroid follicles and foci of papillary carcinoma, most probably representing a papillary carcinoma arising in a struma ovarii. Axial T1-weighted images (3A and 3C) and T2-weighted images with fat saturation (3B and 3D) revealed a multiloculated cystic mass presenting locules with variable signal intensity (“stained glass appearance”) and thick septations. Some locules showed low signal intensity on T1-weighted images and high signal intensity on T2-weighted images (white arrow; 3C and 3D), while other locules exhibited low signal intensity on both T1- and T2-weighted images (white asterisk; 3A and 3B), the latter most probably due to the presence of colloid content. Other areas with intermediate signal on T1- and T2-weighted images (white-bordered white arrow; 3A and 3B) presented linear signal voids (thin arrow; 3B) within it and were compatible with solid areas.. No fatty component was detected. A small amount of ascites was identified in the pouch of Douglas / cul-de-sac (black asterisk; 3B) [GE Signa HDe 1.5T: T1-weighted images (TR=420; TE=12,8); T2-weighted images (TR=2380; TE=105); Proton-density weighted image (TR= 3560; TE= 38,4)].
Figure 4.
Pelvic Magnetic Resonance Imaging. 71-year-old female patient with an ovarian lesion containing thyroid follicles and foci of papillary carcinoma, most probably representing a papillary carcinoma arising in a struma ovarii. Parasagittal (4A), sagittal (4B) and coronal (4C) T2-weighted images, and axial T2-weighted images with fat saturation (4D). Multiple serpiginous vessels presenting as signal voids were seen surrounding (thick white arrow; 4A and 4B) and entering the ovarian mass (thin white arrow; 3A), thus confirming the rich vascularity of the tumor. The left ovary was atrophic, given the postmenopausal age of the patient (black arrow; 4D). A small amount of ascites was detected in the pouch of Douglas / cul-de-sac (black arrow; 4B) [GE Signa HDe 1.5T: T1-weighted image (TR=420; TE=12,8); T2-weighted images (TR=2380; TE=105); Proton-density weighted image (TR=3560; TE=38,4)].
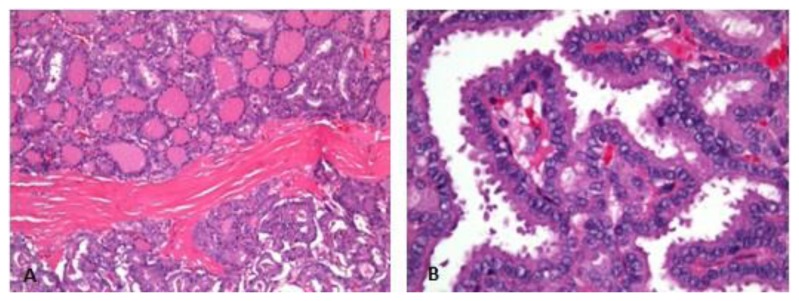
The patient underwent a right salpingo-oophorectomy with no postoperative complications. The right ovary measured 10×9×5cm and weighted 256g; it was partially cystic, with yellow serous fluid and green gelatinous colloid material separated by thick septations. During histological examination of the tumor, thyroid tissue with follicles of variable sizes were detected. A classic pattern of papillary thyroid carcinoma was observed, with no signs of vascular invasion or ovarian capsule invasion (figs. 5A and 5B). The fallopian tube was normal on gross and microscopic observation.
Figure 5.
Microscopic examination of hematoxylin and eosin (h&e) stained sections. 71-year-old female patient with an ovarian lesion containing thyroid follicles and foci of papillary carcinoma, most probably representing a papillary carcinoma arising in a struma ovarii. The ovarian tumor was composed of thyroid follicles and of areas with papillae formation and papillary carcinoma nuclear features (5A), best depicted in the high power view (5B).
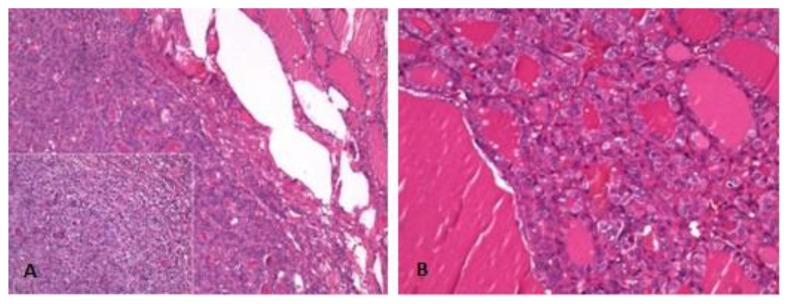
The resection of the remaining thyroid was performed shortly after and a small focus of papillary thyroid carcinoma was detected in the right lobe, surrounded by nodular hyperplasia. This tumor was diagnosed as a follicular variant of papillary thyroid carcinoma, which, once again had no signs of either vascular invasion or extra thyroidal extension (figs. 6A and 6B).
Figure 6.
Microscopic examination of hematoxylin and eosin (h&e) stained sections. 71-year-old female patient with an ovarian lesion containing thyroid follicles and foci of papillary carcinoma, most probably representing a papillary carcinoma arising in a struma ovarii. Low and high power views of left thyroid lobe depict an uncapsulated nodule with oncocytic features and empty and grooved nuclei, respectively (6A). High power view of the right thyroid lobe shows a papillary carcinoma with follicular pattern (6B).
Although no further treatment with ablative I131 therapy was performed, the thyroglobulin levels remained undetectable and there was no evidence of local recurrence or metastatic disease during short-term follow up. However, the patient developed a multiple myeloma during the 2 year follow-up period and died shortly after.
DISCUSSION
Ovarian metastasis of thyroid origin and thyroid carcinoma arising from a struma ovarii are the two major differential diagnoses that have to be considered when a woman with history of metachronous thyroid carcinomas presents with an ovarian mass containing thyroid tissue and foci of thyroid carcinoma.
Awareness of personal and family history of malignant thyroid disease and investigation of thyroid disease through a careful physical examination and thyroid ultrasound are mandatory to differentiate between these two conditions [1–3].
The diagnosis of ovarian metastasis could not be ruled out in this case, since no other teratomatous elements were detected in the ovarian mass and because there was a history of malignant thyroid disease. However, we believe that a papillary thyroid carcinoma arising from a struma ovarii would be the most adequate diagnosis, based mainly on three reasons.
First, thyroid malignancy arising from the struma ovarii is reported in 5%–10% of cases [4], occurring much more commonly than an ovarian metastasis from the thyroid[1, 2]. In the past, many authors did not consider ovarian metastasis as a differential diagnosis when thyroid tissue was identified in an ovarian lesion and other authors dismissed this condition when small thyroid primary tumors were detected or when there was a long time interval between the occurrence of each tumor [2].
Second, papillary carcinoma is the malignant subtype most frequently arising in the struma ovarii and represents 70% of all cases, 44% percent of these being classical type and the remaining 26% being a follicular variant of papillary carcinoma [4, 5]. The concomitant presence of normal thyroid epithelial tissue representing the predominant component in the ovarian lesion, as described in this clinical case, is highly suggestive of struma ovarii origin, even though no other teratomatous elements were identified [2]. Some cases of thyroid-type carcinoma with no benign struma ovarii component have also been described [1].
Third, ovarian metastases from papillary thyroid carcinoma are rare. Papillary carcinomas comprise 80% of clinically detected thyroid cancers and are associated with a favorable prognosis, due to their low metastatic potential, particularly in the absence of extrathyroid or vascular invasion, as was detected in this case [6]. Besides, occult papillary thyroid microcarcinomas were found in 46 out of 408 (11,3 %) thyroid glands during autopsy [7], which suggests that a single focus of microcarcinoma in a thyroidectomy specimen may be an incidental finding without clinical importance. Most of the recurrences of papillary thyroid cancer occur in cervical lymph nodes and its incidence is reported in up to 70% of all cases [8]. Although distant metastases may occur many years or even decades after diagnosis, their prevalence is much lower when compared with follicular carcinomas, and both the lung and the skeleton are the most common sites involved [3]. Only two cases of ovarian metastases with papillary thyroid origin were reported so far in the literature; one presenting as bilateral ovarian metastases in a patient with autoimmune thyroiditis and an aggressive form of primary thyroid malignancy [3] and another presenting as unilateral cystic ovarian metastasis in a patient with widespread metastatic disease [2]. In the unique case of ovarian metastasis from thyroid carcinoma detected 12 years after partial thyroidectomy, the patient also had widespread metastases of a follicular subtype tumor [9]. There was no evidence of cervical or distant metastatic involvement in our patient, during the 12-year follow-up period. Additionally, the variation in histopathology detected between thyroid and ovarian tumors, reduced the likelihood of metastatic involvement [2].
Struma ovarii was first described in 1895 [10] and represents 5% of all ovarian teratomas [4]. It is defined as a monodermal variant of ovarian teratomas in which thyroid tissue is the predominant (>50%) or exclusive component [1, 4]. Moreover, it also includes cases of mature cystic teratomas with macroscopically identifiable thyroid tissue or containing malignant thyroid tissue, even when the thyroid tissue component is less than 50% of the lesion [1].
The term malignant struma ovarii should be avoided and replaced by thyroid-type carcinoma originating in the struma ovarii (specifying the type) to describe this entity, because many cases of strumal carcinoid tumors have been misdiagnosed and reported as malignant struma ovarii [1].
Thyroid carcinoma arising in the struma ovarii is more likely to occur in lesions larger than 16cm or when peritoneal metastatic involvement is identified, the latter being usually described as strumosis [9, 11]. It predominantly affects the left rather than the right ovary, for unexplained reasons [5].
The mean age of presentation is similar to other mature cystic teratomas and is usually between 40 and 60 years of age [1, 4], although some cases have been reported in prepubertal and postmenopausal ages [12, 13]. Most patients present with pelvic pain or irregular menstrual bleeding [4, 11]. Hyperthyroidism is detected in only 5–8% of all cases [4, 5]. Its presence, generally caused by an adenoma, may raise the suspicion for a functional struma ovarii in the absence of goiter [5].
Ascites has been reported in up to 17% of cases and Meigs’ syndrome can occasionally occur [4, 13, 14]. However, tumor cells are usually not present in the peritoneal fluid [4].
Serum CA125 level may be elevated, but this also occurs in association with other germ cell tumors [4].
There are no imaging specific features for struma ovarii. Ultrasound usually reveals a heterogeneous mass with predominantly solid and multiple cystic areas, which reflect the gross pathologic characteristics of this tumor. The visualization of abundant low resistance blood flow within the highly vascular central portion of the tumor has been referred as a typical finding observed with color/Doppler imaging [14, 15]. CT imaging usually reveals a multiloculated cystic mass with different densities among the locules, as well as solid components. In addition, fat and calcifications may be occasionally identified within the tumor, the latter being more easily detected with this technique rather than with MR imaging [10]. Only a few reports of MR imaging findings of struma ovarii are described in the literature. A multiloculated cystic mass presenting a variable signal intensity among the cystic locules (“stained glass appearance”) and a strongly enhancing solid component is regarded as the classic appearance of this entity [16,17]. Locules with very low signal on T1- and T2-weighted images usually contain gelatinous colloid material, which has a higher viscosity than mucinous fluid [16, 17]. The presence of ascites has been also reported [4, 10, 14].
To our knowledge, the imaging appearance of ovarian metastases of thyroid origin was never reported in the literature. Mature cystic teratomas and mucinous neoplasms, either cystadenomas or cystadenocarcinomas, constitute the main differential diagnosis of struma ovarii, according to imaging findings. However, the radiologist should be aware that 50–60% of struma ovarii cases are associated with mature cystic teratomas and, in smaller percentages, with mucinous cystadenomas and carcinoid tumors [1].
Mature cystic teratomas may resemble struma ovarii on ultrasound scans, particularly when they present as a cystic lesion with an echogenic solid component (Rokitansky nodule). However, in opposition to struma ovarii, the blood flow is usually only detected at the periphery of the lesion and the Rokitansky nodule is usually avascular; if the presence of Doppler color is ever detected, the possibility of malignancy transformation should be raised [18, 19]. Mature cystic teratomas can also present as diffuse or partially echogenic masses with sound attenuation (reflecting hair or sebaceous material content) or as cystic masses with multiple linear strands, in relation with the presence of hair [19, 20]. Teeth or wall calcifications are described to occur in 56% of cases and are best depicted on CT. This technique is also very sensitive for the presence of fat, which is reported on 93% of cases ; occasionally, a fat-fluid level may be seen within the cystic lesion [18]. At MR imaging, the intratumoral fat usually shows high signal intensity on T1-weighted images and signal loss on fat saturated T1-weighted images. In addition, the chemical shift artifact can be particularly helpful in the diagnosis of teratomas without demonstrable fat (described as 15% of cases), by demonstrating a drop in signal intensity in the out-of-phase image [20].
Although mucinous tumors usually occur in a different age group, being more prevalent in the sixth and seventh decades of life, their differentiation from struma ovarii can be very difficult at imaging, because both of them most commonly present as multiloculated cystic masses containing fluid of different attenuation or signal intensity on CT and MR imaging, respectively [16, 21]. However, the “stained glass appearance” of mucinous tumors is related to different mucin concentration: thick mucin shows high signal intensity on T1-weighted images and low signal intensity on T2-weighted images, while watery mucin usually shows high signal intensity on T2-weighted images and low signal intensity on T1-weighted images [21]. A malignant cystadenocarcinoma is suspected when solid components or thick and irregular wall and septa are identified, in opposite to benign cystadenomas, which usually have thin and regular wall and septa [21]. Calcifications can be also identified in mucinous tumors and were reported to occur in 34,1% of CT scans [22].
A correct pre-operative diagnosis of struma ovarii is only possible when I131 scintigraphy is performed, in patients with hyperthyroidism and high levels of serum TSH [11].
The majority of patients are diagnosed postoperatively, as was the case of our patient. Macroscopically, the tumor is typically brown or green-brown, solid or predominantly solid and gelatinous. Nonetheless, cystic or predominantly cystic multilocular tumors have also been described [1, 4].
Thyroid carcinoma arising in a struma ovarii is reported to occur in 5–10% of cases [4]. The morphological criteria of these tumors is based on classical criteria for primary thyroid carcinomas [1]. The presence of ground glass overlapping nuclei, nuclear grooves and papillary architecture are diagnostic of papillary carcinomas, which represent the majority of thyroid carcinomas arising in struma ovarii. The identification of psammoma bodies is also highly suspicious [1]. Lesions sharing the same nuclear features but lacking papillary architecture represent the follicular variant of papillary carcinoma [1]. Follicular carcinoma is encountered in 30% of cases of thyroid carcinomas arising in struma ovarii [5]. Its diagnosis is more difficult, since there is no capsule in the ovarian counterpart and evidence of malignancy is only suggested by vascular invasion, metastases or invasion into the remaining ovarian tissue [1, 4]. A new variant of follicular carcinoma named highly differentiated follicular carcinoma of ovarian origin, has been recently described [1] and is characterized by extraovarian dissemination of thyroid elements [4].
Surgery is mandatory for diagnosis, surgical staging and definite treatment [11]. The surgical approach depends on patient age, with unilateral salpingo-oophorectomy being recommended in younger women in reproductive age and total abdominal hysterectomy and bilateral salpingo-oophorectomy being performed in postmenopausal women [11]. However, the management of struma ovarii cases after surgery is still not clearly defined, as it is based on small series review [4]. Some authors suggest that the treatment should be similar to its thyroid counterpart [23]. Total thyroidectomy is recommended to provide effective ablative therapy with I131 and levothyrotoxine suppressive therapy. However, other authors recommend a similar management as used in other germ cell tumors [4].
The prognosis of thyroid-type carcinoma is unknown, owing to its rareness and to the absence of consensus in both its diagnosis and treatment. A stratification of malignancy into high and low risk patients was suggested [24]. A focus of thyroid carcinoma with less than 2 cm is considered as low risk, with larger carcinomas, extraovarian disease and aggressive histological features being considered as high-risk features [4]. However, most cases do not have a clinical aggressive course; therefore, the detection of malignancy in struma ovarii does not necessarily correlate with biological behavior [1].
Although there are no standard follow-up protocols for these patients, a 10-year follow-up is recommended and includes thyroglobulin levels assessment or a I131 whole-body scan. The average time for recurrence is 4 years and is reported in 15% of cases [4]. Metastases are uncommonly seen in these patients, occurring in less than 23% of cases [4, 5]. Follicular carcinoma is more likely to spread to distant organs, namely the lung, liver and central nervous system, while papillary carcinoma usually extends to the abdominal cavity, lymph nodes and, rarely, to the liver [4]. To our knowledge, metastatic involvement of the thyroid gland has never been reported in the literature.
TEACHING POINT
The awareness of malignant thyroid disease is mandatory when thyroid tissue and thyroid-subtype carcinoma are detected within an ovarian mass. Two major differential diagnoses must be considered: metastatic carcinoma of thyroid origin and thyroid carcinoma arising in a struma ovarii. The identification of a multiloculated ovarian cystic mass presenting a solid component and a variable signal intensity between locules (“stained glass appearance”) on MR imaging, should raise the suspicion of struma ovarii.
Table 1.
Summary table of thyroid carcinoma arising in struma ovarii
| Etiology | Unknown |
| Incidence | Struma ovarii: 5% of all ovarian teratomas |
| Thyroid-carcinoma arising in struma ovarii: 5–10% Papillary carcinoma - 70% (classical subtype - 44%; follicular variant subtype -26%) Follicular carcinoma - 30% | |
| Gender ratio | Women |
| Age predilection | 4th–6th decade |
| Risk factors | Lesions> 16cm; Strumosis |
| Treatment | Unilateral or bilateral salpingo-oophorectomy (depending on age) + total thyroidectomy |
| Prognosis | Still unknown |
| Findings on imaging |
|
Table 2.
Differential diagnosis of struma ovarii
| US | CT | MR | |
|---|---|---|---|
| Struma ovarii |
|
|
|
| Mucinous tumors (mucinous cystadenoma or cystadenocarcinoma) |
|
|
|
| Mature cystic teratomas |
|
|
|
ABBREVIATIONS
- CT
Computed Tomography
- MR
Magnetic Resonance
- US
Ultrasound
REFERENCES
- 1.Roth LM, Talerman A. The enigma of struma ovarii. Pathology. 2007 Feb;39(1):139–46. doi: 10.1080/00313020601123979. [DOI] [PubMed] [Google Scholar]
- 2.Logani S, Baloch ZW, Snyder PJ. Cystic ovarian metastasis from papillary thyroid carcinoma: a case report. Thyroid. 2001 Nov;11(11):1073–5. doi: 10.1089/105072501753271789. [DOI] [PubMed] [Google Scholar]
- 3.Brogioni S, Viacava P, Tomisti L. A special case of bilateral ovarian metastases in a woman with papillary carcinoma of the thyroid. Exp Clin Endocrinol Diabetes. 2007 Jun;115(6):397–400. doi: 10.1055/s-2007-973853. [DOI] [PubMed] [Google Scholar]
- 4.Salman W, Singh M, Twaij Z. A case of papillary thyroid carcinoma in struma ovarii and review of the literature. Patholog Res Int. 2010 Aug 2;2010:352476. doi: 10.4061/2010/352476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomee JF, Van der Heijden PF, Van den Hout JH. Papillary carcinoma in struma ovarii: an unusual presentation. Neth J Med. 2008 Jun;66(6):248–51. [PubMed] [Google Scholar]
- 6.Mazzaferri EL, Massoll N. Management of papillary and follicular (differentiated) thyroid cancer: new paradigms using recombinant human thyrotropin. Endocr Relat Cancer. 2002 Dec;9(4):227–47. doi: 10.1677/erc.0.0090227. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto Y, Maeda T, Izumi K. Occult papillary carcinoma of the thyroid. A study of 408 autopsy cases. Cancer. 1990 Mar 1;65(5):1173–9. doi: 10.1002/1097-0142(19900301)65:5<1173::aid-cncr2820650524>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Leboulleux S, Rubino C, Baudin E. Prognostic Factors for Persistent or Recurrent Disease of Papillary Thyroid Carcinoma with Neck Lymph Node Metastases and/or Tumor Extension beyond the Thyroid Capsule at Initial Diagnosis. J Clin Endocrinol Metab. 2005 Oct;90(10):5723–9. doi: 10.1210/jc.2005-0285. [DOI] [PubMed] [Google Scholar]
- 9.Young RH, Jackson A, Wells M. Ovarian metastasis from thyroid carcinoma 12 years after partial thyroidectomy mimicking struma ovarii: report of a case. Int J Gynecol Pathol. 1994 Apr;13(2):181–5. doi: 10.1097/00004347-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Van de Moortele K, Vanbeckevoort D, Hendrickx S. Struma ovarii: US and CT findings. JBR-BTR. 2003 Jul-Aug;86(4):209–10. [PubMed] [Google Scholar]
- 11.Janszen EW, Van Doorn HC, Ewing PC. Malignant struma ovarii]: good response after thyroidectomy and I ablation therapy. Clin Med Oncol. 2008;2:147–52. doi: 10.4137/cmo.s410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezon I, Zilbert N, Pinkney L. A large struma ovarii tumor removed via laparoscopy in a 16-year-old adolescent. J Pediatr Surg. 2007 Aug;42(8):E19–22. doi: 10.1016/j.jpedsurg.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Devaney K, Snyder R, Norris HJ. Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int J Gynecol Pathol. 1993 Oct;12(4):333–43. doi: 10.1097/00004347-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez DM, Lee V, Bhatt S. Struma ovarii with papillary thyroid carcinoma. J Clin Imaging Sci. 2011;1:44. doi: 10.4103/2156-7514.84322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zalel Y, Seidman DS, Oren M. Sonographic and clinical characteristics of struma ovarii. J Ultrasound Med. 2000 Dec;19(12):857–61. doi: 10.7863/jum.2000.19.12.857. [DOI] [PubMed] [Google Scholar]
- 16.Matsuki M, Kaji Y, Matsuo M. Struma ovarii: MRI findings. Br J Radiol. 2000 Jan;73(865):87–90. doi: 10.1259/bjr.73.865.10721328. [DOI] [PubMed] [Google Scholar]
- 17.Dohke M, Watanabe Y, Takahashi A, et al. Struma ovarii: MR findings. J Comput Assist Tomogr. 1997 Mar-Apr;21(2):265–7. doi: 10.1097/00004728-199703000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Zalel Y, Caspi B, Tepper R. Doppler flow characteristics of dermoid cysts: unique appearance of struma ovarii. J Ultrasound Med. 1997 May;16(5):355–8. [PubMed] [Google Scholar]
- 19.Outwater EK, Siegelman ES, Hunt JL. Ovarian Teratomas: Tumor Types and Imaging Characteristics. Radiographics. 2001 Mar-Apr;21(2):475–90. doi: 10.1148/radiographics.21.2.g01mr09475. [DOI] [PubMed] [Google Scholar]
- 20.Park SB, Kim JK, Kim KR. Imaging findings of complications and unusual manifestations of ovarian teratomas. Radiographics. 2008 Jul-Aug;28(4):969–83. doi: 10.1148/rg.284075069. [DOI] [PubMed] [Google Scholar]
- 21.Jung SE, Lee JM, Rha SE. CT and MR Imaging of Ovarian Tumors with Emphasis on Differential Diagnosis. Radiographics. 2002 Nov-Dec;22(6):1305–25. doi: 10.1148/rg.226025033. [DOI] [PubMed] [Google Scholar]
- 22.Okada S, Ohaki Y, Inoue K. Calcifications in mucinous and serous cystic ovarian tumors. J Nippon Med Sch. 2005 Feb;72(1):29–33. doi: 10.1272/jnms.72.29. [DOI] [PubMed] [Google Scholar]
- 23.Mattucci M, Dellera A, Guerriero A. Malignant struma ovarii: a case report and review of the literature. J Endocrinol Invest. 2007 Jun;30(6):517–20. doi: 10.1007/BF03346337. [DOI] [PubMed] [Google Scholar]
- 24.Yassa L, Sadow P, Marqusee E. Malignant struma ovarii. Nat Clin Pract Endocrinol Metab. 2008 Aug;4(8):469–72. doi: 10.1038/ncpendmet0887. [DOI] [PubMed] [Google Scholar]