Abstract
Extracellular superoxide dismutase (SOD3) is highly expressed in renal tissues and an important regulator of vascular function. We hypothesized that deletion of SOD3 will attenuate recovery of renal blood flow and increase oxidative stress and injury following renal ischemia/reperfusion.
Expression and activities of SOD isoforms, basal superoxide production, and NADPH oxidase activity were evaluated in renal cortical homogenates from male and female wild-type (WT) and SOD3-knockout (SOD3−/−) mice. After 60 minutes of unilateral ischemia, we assessed renal reperfusion using an ultrasonic flow probe (Transonic) and histological measures of oxidative stress and injury in the post-ischemic kidneys.
Total SOD activity was reduced in male SOD3−/− versus WT mice (7.4±0.3 vs 12.6±1.7 U/ml, p=0.01). However, total SOD activity was similar in female SOD3−/− versus WT mice (14.2±1.4 vs 16.3±1.8 U/ml, n.s.), suggesting up-regulated SOD1 activity. Basal superoxide production was significantly higher in female SOD3−/− versus WT counterparts, but not male. NADPH oxidase activity was similar in WT and SOD3−/− mice. Compared to WT mice, renal blood flow after ischemia was attenuated in kidneys from male, but not female, SOD3−/− mice. After 24 hours, kidneys from male and female SOD3−/− mice demonstrated more oxidative stress (3-nitrotyrosine staining) and renal cast formation than those from WT mice.
Loss of SOD3 impairs reperfusion after renal ischemia in male but not in female mice, which is associated with preserved total SOD activity in females. Yet, SOD3 deletion increases post-ischemic oxidative stress and injury in both sexes, demonstrating a key role for SOD3 in renal ischemia/reperfusion injury.
Keywords: kidney, ischemia, reperfusion, reperfusion injury, renal circulation, oxidative stress, superoxide, superoxide dismutase
Ischemia/reperfusion (I/R) injury is an important cause of acute kidney injury (AKI) and delayed graft function after kidney transplantation. Experimental models have provided convincing evidence for a key role of reactive oxygen species (ROS) in renal I/R injury1–3. In this setting, the major sources of superoxide, the most upstream molecule within the group of ROS, seem to be NADPH oxidase, enzymes of the mitochondrial respiratory chain and xanthine oxidase2, 4–5. In humans with AKI, the T allele of the C242T gene polymorphism of NADPH oxidase is associated with increased markers of oxidative stress and increased risk of dialysis and death6. Thus, there is compelling clinical evidence for a role of superoxide in the pathogenesis of AKI.
Superoxide reacts with nitric oxide (NO) at a near-diffusion limited rate to form peroxynitrite, which mediates several aspects of renal I/R injury3. The superoxide dismutases (SOD) are a natural defense against oxidative injury because these enzymes dismutate superoxide to hydrogen peroxide, which is subsequently detoxified by catalase or glutathione peroxidase. Induction or administration of SOD might therefore be a potential therapeutic approach to limit oxidative stress and/or injury in renal I/R. Three isoforms of SOD exist: cytoplasmic or Cu/Zn SOD (SOD1), mitochondrial or Mn SOD (SOD2) and extracellular SOD (SOD3), but the precise role of the various isoforms in renal I/R have not been studied.
It has been known for several decades that the post-ischemic kidney is characterized by endothelial cell-swelling and absent or delayed reflow7, but the pathogenic role of the endothelium has only more recently been appreciated. Studies have demonstrated that post-ischemic renal vasoconstriction is associated with a profound impairment of endothelium-dependent, NO-mediated vasodilation in the renal vasculature8–12. Furthermore, improving endothelial function and reducing post-ischemic renal vasoconstriction improves renal outcome9, 13–14. Of the three isoforms, SOD3 is the most widely expressed isoform in the kidney and in the vasculature, and thus might be considered the most relevant isoform for protection against ischemia-induced changes in the kidney, including the maintenance of renal blood flow (RBF)15–16. In addition, lack of SOD3 impairs vascular function in other settings, as demonstrated in the angiotensin II-dependent hypertension model and in the neovascularization response after hindlimb ischemia17–18.
Sex differences may also modulate the susceptibility of various organs to I/R injury. As examples, men are more susceptible to kidney I/R than women19 and male mice have increased susceptibility to ischemic injury compared to female mice20–21. This sex difference seems related to differential activation of post-ischemic signaling pathways including activation of NO-synthase and modulation of antioxidant enzyme expression and activity21–23. Because SOD3 is expressed in high levels within the kidney and the vasculature, we hypothesized that lack of SOD3 would impair renal reperfusion, increase oxidative stress, and increase renal injury in a mouse model of renal I/R. In light of potential sex differences in the regulation of antioxidant enzymes, we also compared male and female WT and SOD3−/− mice in this study.
Results
Expression and activity of SOD isoforms
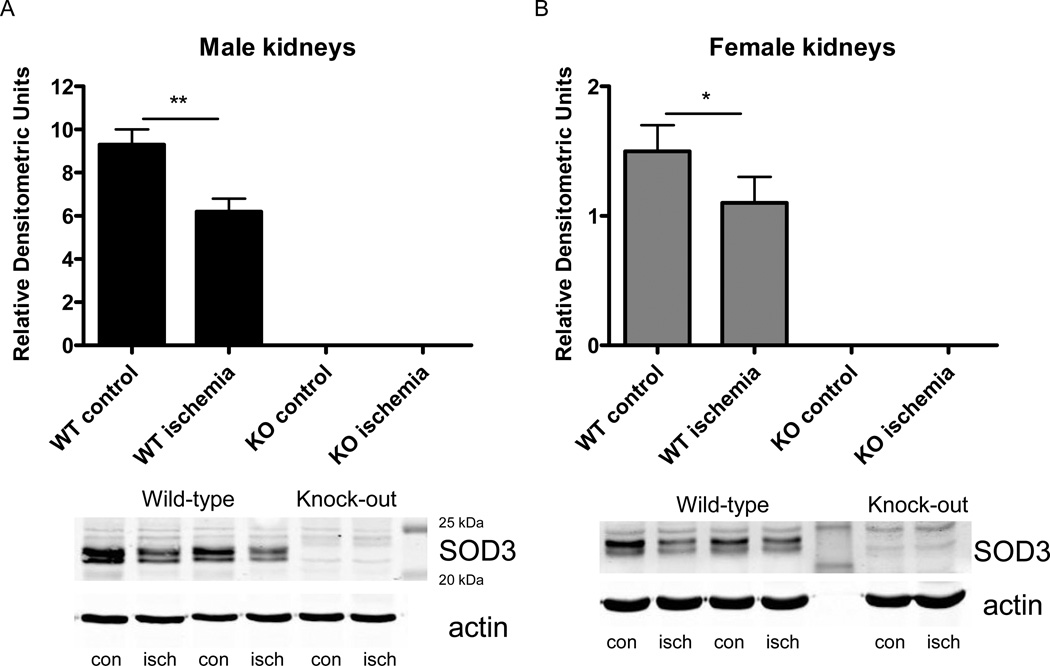
As expected, SOD3 expression was absent in male and female SOD3−/− mice (figure 1). In both male and female WT mice, SOD3 expression in the renal cortex was lower in post-ischemic kidneys after 1 hour of reperfusion compared to control kidneys (figure 1). Further, SOD1 and SOD2 expression did not differ between ischemic and control kidneys and between WT and SOD3−/− mice of both sexes (data not shown).
Figure 1. SOD3 expression in kidneys from male and female WT and SOD3−/− mice.
Expression of SOD3 in the cortex of paired control and post-ischemic kidneys after 1 hour of reperfusion of male (A) and female (B) WT and SOD3−/− mice (n=7 in WT males and n=7 in WT females, respectively). Bottom panel shows representative blot along with molecular weight standards. cont, control kidney; isch, ischemic kidney; * p<0.05, ** p<0.01.
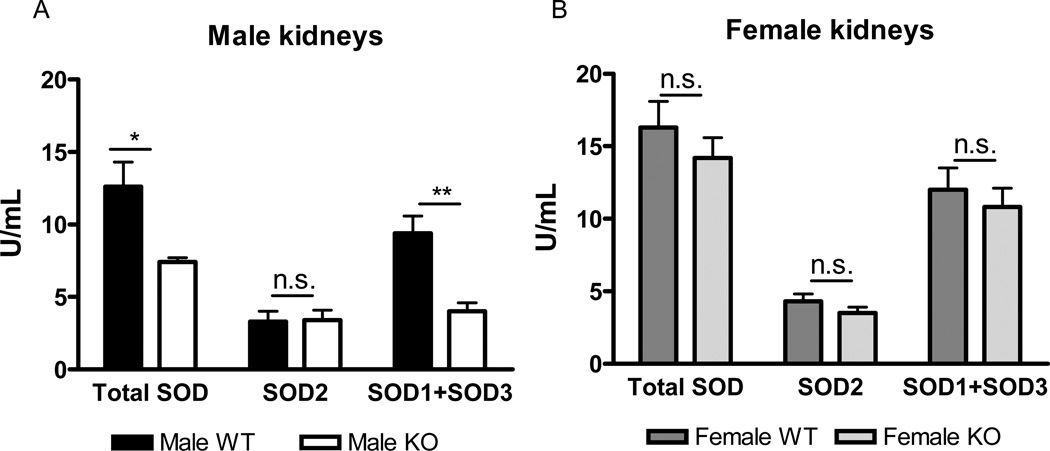
Total SOD activity was lower in kidneys of male SOD3−/− mice versus male WT mice (figure 2 A). Inhibition of the cyanide-sensitive isoforms, SOD1 and SOD3, revealed that the lower total SOD activity in male SOD3−/− mice was not due to differences in SOD2 activity, but due to lower SOD1+SOD3 activity. Since we verified SOD3 expression is absent, then the lower total SOD activity seen in the renal cortex of male control kidneys is due to the loss of SOD3.
Figure 2. SOD isoform activities in kidneys from male and female WT and SOD3−/− mice.
SOD isoform activities in the cortex of kidneys from male (A) and female (B) WT and SOD3−/− mice (n=8 in male WT, n=7 in male SOD3−/−, n=8 in female WT and n=8 in female SOD3−/− mice, respectively). n.s., not significant, (p>0.05), * p<0.05, ** p<0.01
In females, loss of SOD3 did not affect total SOD, SOD2 or SOD1+SOD3 activities (figure 2 B). This indicates that that there was compensatory upregulation of SOD1 activity in female SOD3−/− mice, or alternatively, that there is limited contribution of SOD3 activity to total SOD activity in females.
Basal superoxide production and NADPH oxidase activity
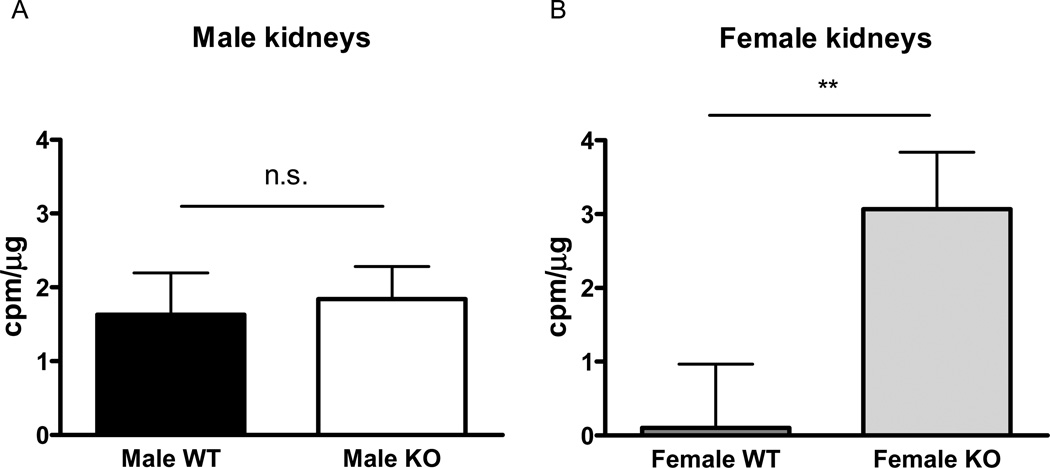
In renal cortical homogenates of kidneys from male and female mice, basal superoxide production was higher in the female, but not in the male, SOD3−/− mice compared to WT controls (figure 3 A and B). Renal cortical homogenates from post-ischemic kidneys after 1 hour of reperfusion did not display an increase in basal superoxide production between male SOD3−/− and WT mice (2.0±1.3 vs 2.9±1.3 cpm/µg protein, n.s), and was also similar between female SOD3−/− and WT mice (2.8±2.8 vs 1.6±1.1 cpm/µg, n.s.).
Figure 3. Basal superoxide production in kidneys from male and female WT and SOD3−/− mice.
Basal superoxide production in the cortex of kidneys from male (A) and female (B) WT and SOD3−/− mice (n=6 in male WT, n=8 in male SOD3−/− mice, n=8 in female WT, n=7 in female SOD3−/− mice, respectively). n.s., not significant, (p>0.05), * p<0.05
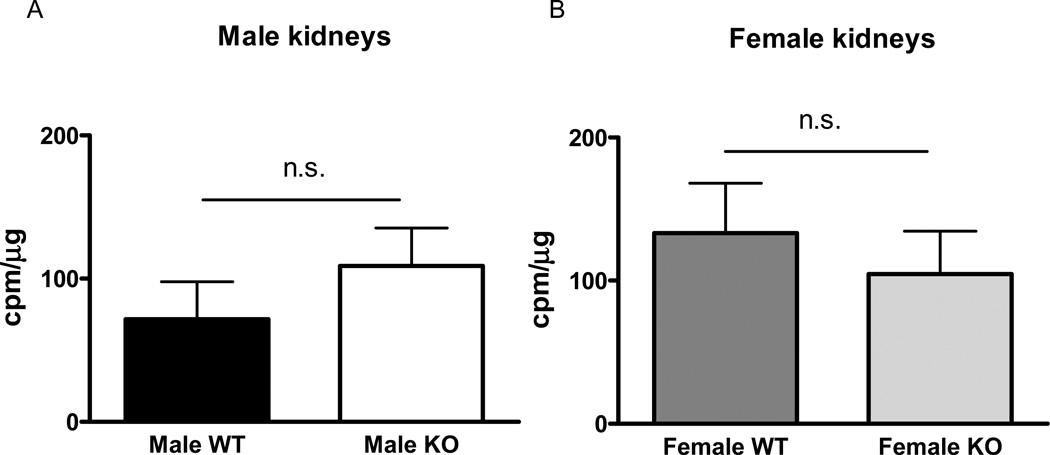
NADPH oxidase activity was similar between SOD3−/− and WT mice in both the male and female mice strains (figure 4 A and B). Ischemia did not affect NADPH oxidase production in any of the groups, and was therefore similar between male SOD3−/− and WT mice (65±53 vs 39±25 cpm/µg protein, n.s), and between female SOD3−/− and WT mice (122±72 vs 70±61 cpm/µg, n.s.).
Figure 4. NADPH oxidase activity in kidneys from male and female WT and SOD3−/− mice.
NADPH oxidase activity in the cortex of kidneys in male (A) and female (B) WT and SOD3−/− mice (n=6 in male WT, n=8 male SOD3−/− mice, n=8 female WT, n=7 female SOD3−/− mice, respectively). n.s., not significant, (p>0.05), * p<0.05
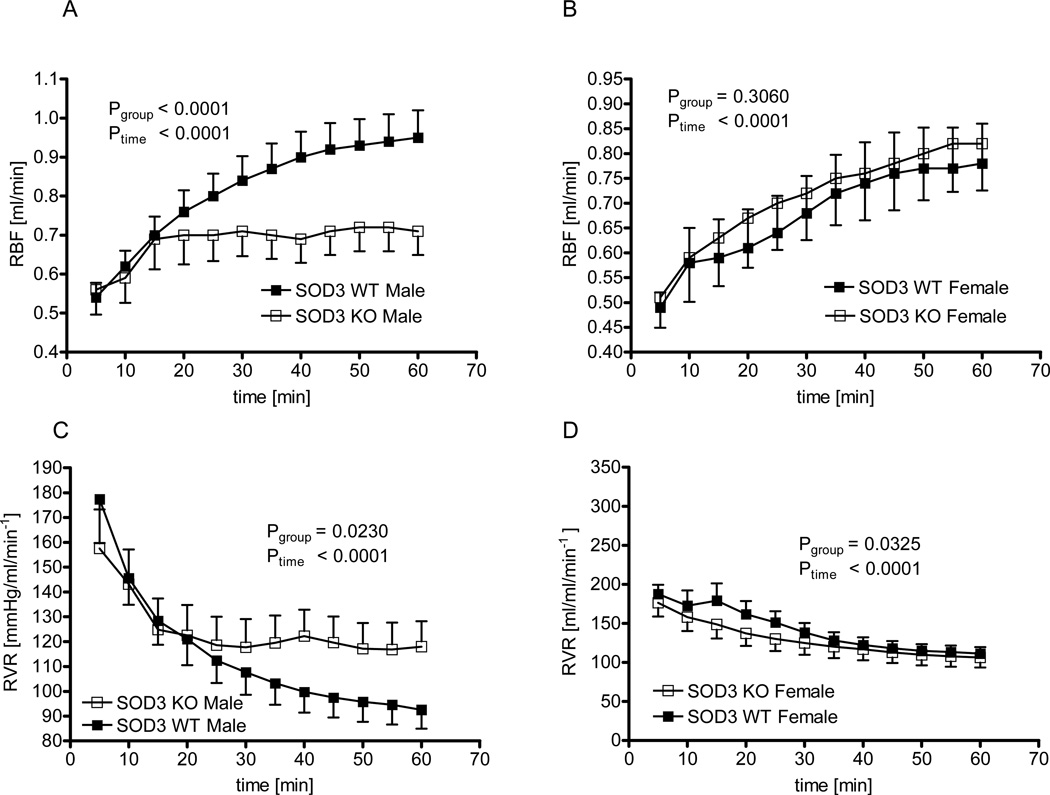
Renal blood flow
Before the induction of ischemia, baseline RBF was similar in male WT versus male SOD3−/− mice (1.36±0.07 versus 1.39±0.15 ml/min, p=0.86) and in female WT versus female SOD3−/− mice (1.02±0.09 versus 1.10±0.12 ml/min, p=0.56). In male WT mice, RBF returned to 56±3% of baseline 20 minutes following reperfusion, and reached a maximum of 70±4% 60 minutes after removing the renal artery clamp (figure 5 A for absolute values). In male SOD3−/− mice, RBF returned to 54±5% at 20 minutes but showed an attenuated increase thereafter resulting in a maximum of 57±6% after 60 minutes. Thus, RBF recovery was significantly blunted in male SOD3−/− mice compared to male WT animals (p<0.0001 by 2-way ANOVA). Similar results were found when renal vascular resistance was examined. The progressive post-ischemic reduction of renal vascular resistance was attenuated in male SOD3−/− versus WT mice (p=0.023 by 2-way ANOVA, figure 5 C).
Figure 5. Renal blood flow recovery in male and female WT and SOD3−/− mice.
Renal blood flow after 60 minutes of ischemia in male (A) and female (B) WT and SOD3−/− mice, and renal vascular resistance in male (C) and female (D) WT and SOD3−/− mice (n=16 in WT male, n=13 in SOD3−/− male, n=16 in female WT and n=13 in female SOD3−/− mice).
In striking contrast to the findings in male mice, female SOD3−/− mice showed no impairment in renal reperfusion compared to WT animals. In female WT mice, RBF was 59±2% of baseline 20 minutes following reperfusion and reached a maximum of 78±5% after 60 minutes of reperfusion (figure 5 B for absolute values). Female SOD3−/− mice showed a similar return of RBF of 61±4% at 20 minutes and a maximum of 77±5% after 60 minutes reperfusion. Thus, there was no difference in return of RBF between female SOD3−/− and WT mice (2-way ANOVA). When examining the post-ischemic course of renal vascular resistance, the reduction in renal vascular resistance was initially even more pronounced in female SOD3−/− than in female WT mice (figure 5 D), and thus the opposite from the response in male animals (figure 4 C).
Blood pressure under anesthesia was slightly lower in the male SOD3−/− than in the WT mice, which remained stable during ischemia and then slightly dropped during reperfusion (Male WT vs male SOD3−/− mice: 85±4 vs 79±5 mmHg at baseline, 86±5 vs 79±4 during ischemia, 81±7 vs 77±4 during reperfusion; 2-way ANOVA: Ptime= 0.01, Pgenotype< 0.01, Pinteraction= 0.53). Blood pressure showed a very similar pattern in the female mice, being slightly lower in the SOD3−/− mice (Female WT vs female SOD3−/− mice: 88±5 vs 78±5 mmHg at baseline, 87±5 vs 77±6 during ischemia, 84±6 vs 74±5 during reperfusion; 2-way ANOVA: Ptime= 0.02, Pgenotype< 0.01, Pinteraction= 0.87). Thus, the pattern of blood pressure change during the surgical procedure was similar in the male and female groups, and can therefore not account for the different RBF responses.
Histological markers of oxidative stress
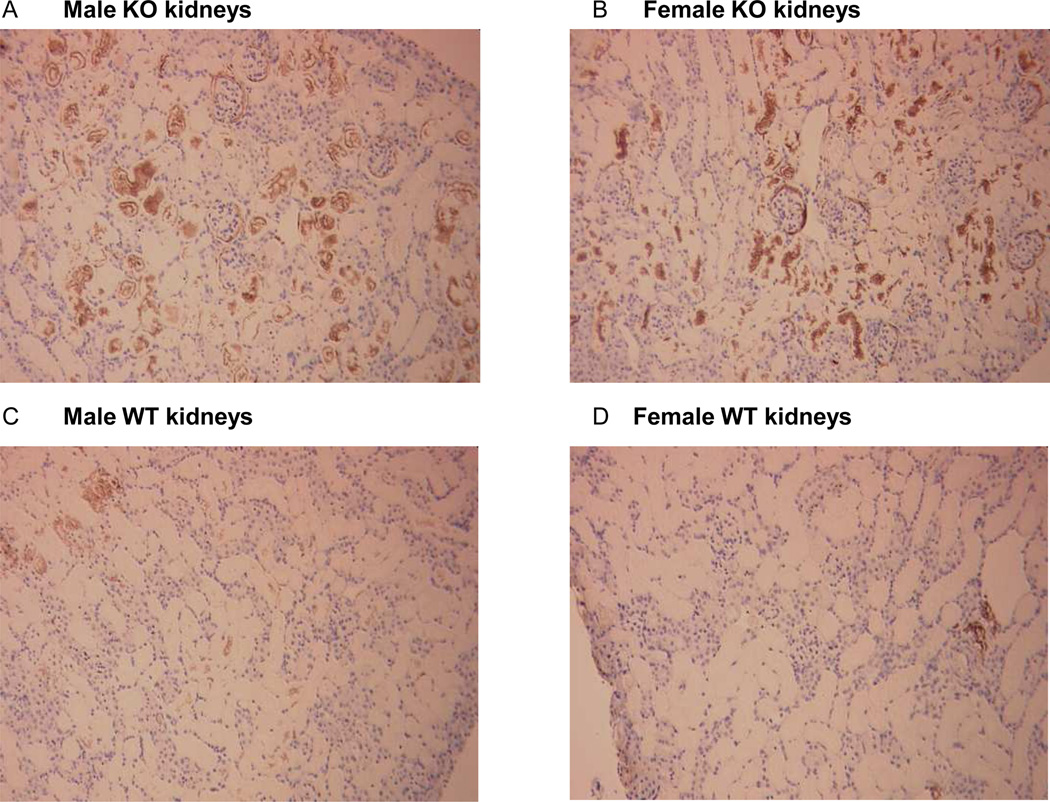
Oxidative stress in control and post-ischemic kidneys was assessed by staining for 3-nitrotyrosine (3-NT), a marker of peroxynitrite formation, and for 8-hydroxy-2'deoxyguanosine (8-OHdG), a marker of oxidative modification of DNA. While there was no difference in the control kidneys for 3-NT staining, post-ischemic kidneys after 1 hour of reperfusion from male animals showed the greatest intensity for 3-NT (from 0=nil staining to 3 strong staining, figure S1): 2.5±0.2 in male SOD3−/− mice, 2.3±0.5 in male WT, 1.6±0.3 in female SOD3−/− mice, 1.4±0.2 in female WT mice (2-way ANOVA: Psex=0.03, Pgenotype=0.54, Pinteraction= 0.95). For 8-OHdG, greatest staining intensity was found in male SOD3−/− mice (figure S2): 2.2±0.4 in male SOD3−/− mice, 1.1±0.7 in male WT, 1.3±0.8 in female SOD3−/− mice, 1.4±0.6 in female WT (2-way ANOVA: Psex= 0.27, Pgenotype= 0.06, Pinteraction= 0.04). Most interestingly, after 24 hours of reperfusion, 3-NT staining was higher in the SOD3−/− mice than in the WT mice (figure 6): 2.5±0.3 in male SOD3−/− mice, 0.6±0.3 in male WT, 1.8±0.5 in female SOD3−/− mice, 0.3±0.2 in female WT mice (2-way ANOVA: Psex=0.21, Pgenotype<0.001, Pinteraction=0.55).
Figure 6. Nitrotyrosine staining in post-ischemic kidneys.
Representative 3-nitrotyrosine staining after 24 hours of reperfusion in the cortex of post-ischemic kidneys of male (A) and female (B) SOD3−/− mice and male (C) and female (D) WT mice
Renal damage and inflammation
In our unilateral model of renal ischemia, and with a functioning contralateral non-ischemic kidney, post-ischemic serum creatinine values were not elevated and were not different across the groups (after 1 hour of reperfusion: Male WT versus SOD3−/− mice: 1.4±0.5 mg/dl vs 1.4±0.4 mg/dl, n.s.; and female WT versus SOD3−/− mice: 1.3±0.4 mg/dl vs 1.3±0.3 mg/dl, n.s; after 24 hours: 1.0±0.1 mg/dl vs 0.9±0.2 mg/dl, n.s.; and 0.9±0.1 mg/dl vs 0.9±0.1 mg/dl, n.s.) The outcome of the post-ischemic kidneys was assessed by histology after 1 and after 24 hours of reperfusion. As expected in renal I/R injury, the most severe lesions were noted within the renal medulla, and no injury was seen in the non-ischemic control kidneys, confirming the absence of any potential injury related to the surgical procedure.
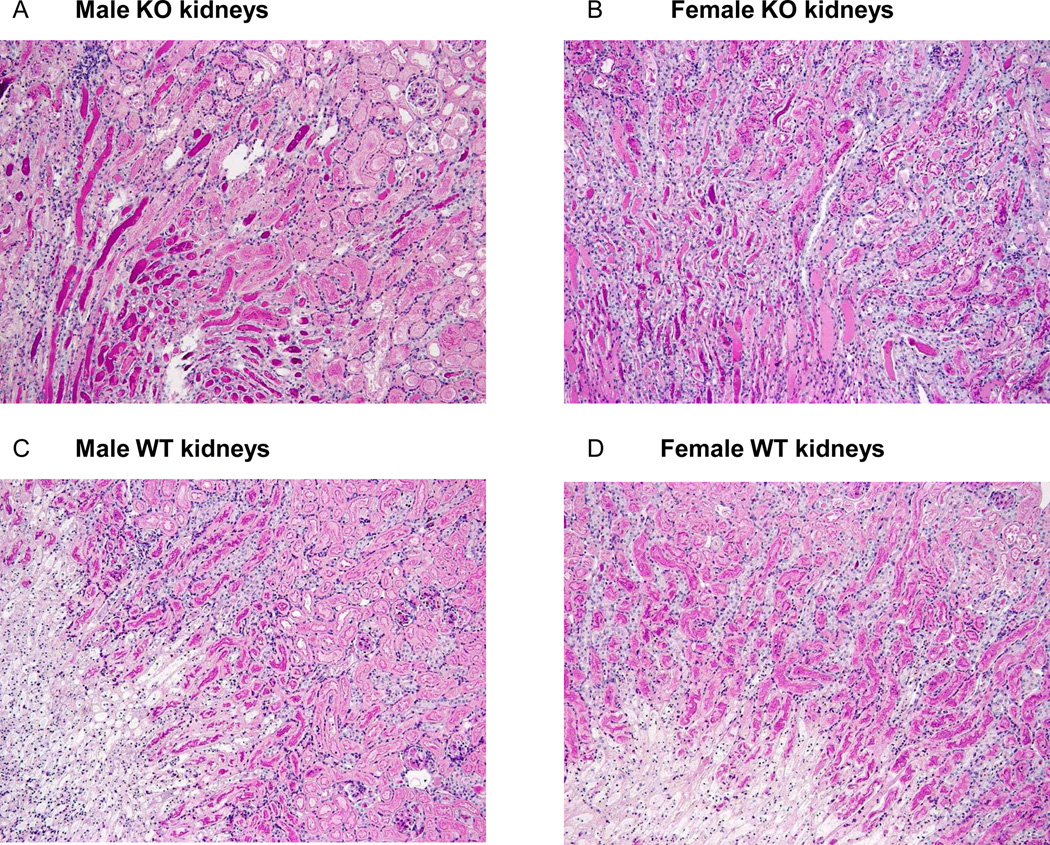
No difference in renal injury was found between groups immediately after 1 hour of reperfusion (data not shown). After 24 hours of reperfusion, tubular necrosis within the outer and inner medulla did not differ between the groups (table 1). Infiltration by multi-nucleated cells as assessed by PAS staining was found to be lower in the SOD3−/− mice in the inner medulla (2-way ANOVA: Psex= 0.13, Pgenotype= 0.02, Pinteraction= 0.45). To confirm whether there is any difference in the infiltration by immune cells we stained the sections for macrophages (F4/80) and T-cells (CD-3). We detected a trend towards more T-cell infiltration in the medulla of SOD3−/− mice (2-way ANOVA: Psex= 0.36, Pgenotype= 0.08, Pinteraction= 0.85) (table S1). Most strikingly, tubular cast formation was significantly increased in the SOD3−/− mice compared to WT mice (Outer medulla: Psex= 0.40, Pgenotype= 0.0002, Pinteraction= 0.74; Inner medulla: Psex= 0.39, Pgenotype= 0.004, Pinteraction= 0.39 by 2-way ANOVA) (figure 7).
Table 1.
Renal injury in post-ischemic kidneys
| Male WT | Male SOD3−/− | Female WT | Female SOD3−/− | ||
|---|---|---|---|---|---|
| Outer Medulla | Tubular necrosis | 4.0±0.0 | 3.8±0.2 | 3.7±0.2 | 3.8±0.2 |
| Cell infiltration | 1.5±0.2 | 1.9±0.3 | 1.0±0.0 | 1.5±0.2 | |
| Tubular casts | 1.0±0.0 | 2.3±0.4 | 1.2±0.2 | 2.4±0.3 | |
| Inner Medulla | Tubular necrosis | 4.0±0.0 | 2.9±0.4 | 3.8±0.2 | 3.4±0.3 |
| Cell infiltration | 2.8±0.2 | 2.2±0.3 | 3.0±0.0 | 2.6±0.2 | |
| Tubular casts | 1.0±0.0 | 2.2±0.4 | 1.0±0.0 | 1.6±0.3 |
Renal injury scores in post-ischemic kidneys after 24 hours of reperfusion in male WT (n=6) and SOD3−/− (n=8) mice and female WT (n=6) and SOD3−/− (n=9) mice (0=nil, 1=mild, 2=moderate, 3=severe and 4=very severe changes).
Figure 7. Tubular cast formation in post-ischemic kidneys.
Representative tubular cast formation after 24 hours of reperfusion in post-ischemic kidneys of male (A) and female (B) SOD3−/− mice and male (C) and female (D) WT mice (PAS staining, original magnification ×100)
Discussion
The current study demonstrates that post-ischemic recovery of RBF is impaired in male mice with deletion of SOD3. Surprisingly, this was not observed in female SOD3−/− mice, and was associated with preserved total SOD activity in these animals. Renal injury after 24 hours of reperfusion, however, was aggravated in both male and female SOD3−/− mice compared to WT controls, and this was associated with increased oxidative stress. Our study therefore demonstrates that SOD3 is protective in renal I/R injury, but also that the effects of SOD3 deletion on various aspects of the renal I/R model are significantly modified by sex.
Each of the three SOD isoforms has been demonstrated to have protective effects in a wide range of conditions, but SOD3, being widely expressed in the cardiovascular system and in the kidney, has attracted substantial attention15–16. Loss of SOD3 exaggerates angiotensin II-dependent hypertension, which is associated with increased oxidative stress and endothelial dysfunction in resistance vessels17. In addition, post-ischemic neovascularization in the hindlimb circulation is impaired in mice lacking SOD318. High levels of mRNA and strong staining for SOD3 protein has been demonstrated in the mouse kidney, in particular in the cytoplasm of cortical tubular cells15. Furthermore, strong expression was found in mouse and human vessels15–16. Because of this pattern of expression and the association between renal vascular function and renal outcome after I/R injury9, 13, we studied the role of SOD3 deletion in renal ischemia.
We found that sex, genotype, and ischemia had no effect on SOD1 and SOD2 protein expression. In contrast, SOD3 expression decreased in the ischemic compared to the non-ischemic kidneys of male and female WT mice. Because the catalytic activity of the SODs can be affected independently of expression levels17, we also examined levels of SOD activity. In the control kidneys of male SOD3−/− mice, total SOD activity was reduced based on a reduction in SOD1+SOD3 activity, indicating that there was no compensatory increase of SOD1 activity. In control kidneys of female SOD3−/− mice, total and SOD1+SOD3 activity were not reduced. This indicates that there was either compensatory upregulation of SOD1 in female SOD3−/− mice or little dependence of total SOD activity on SOD3 activity in females. The former explanation, a compensatory upregulation of SOD1 in female SOD3−/− mice appears much more likely, because the abundant expression of SOD3 in the female WT mice suggest that this isoform contributes to total SOD activity in females. During ischemia we found no change in SOD activities, which is partly in contrast to studies in rats, reporting a decrease in SOD1 and SOD3 and an increase in SOD2 expression and activity after renal I/R24–25. In male mice, a previous study demonstrated no acute changes in any of the three isoforms, although a bilateral model of ischemia had been used22. Differences in the regulation of SOD isoforms between species and/or between different ischemia protocols are therefore likely.
We also determined basal production of superoxide and NADPH oxidase activity in renal cortical homogenates. Interestingly, basal superoxide production was increased in female, but not in the male SOD3−/− mice. NADPH oxidase activity, however, was not increased in male or female SOD3−/− mice compared to their WT counterparts suggesting that this activity is not involved in the pro-oxidant capacity. Differential regulation of other pro-oxidant enzymes, such as xanthine oxidase or mitochondrial sources of superoxide, and/or differences in anti-oxidant enzymes including catalase and glutathione peroxidase, not examined in the current study, might have contributed to this difference.
We then studied the effects of SOD3 deletion on the recovery of RBF, which has been shown to be important for recovery of renal function after an ischemic episode9, 11. Recovery of RBF after ischemia was attenuated in male, but not in female, SOD3−/− mice. This is in line with the aforementioned preserved total SOD activity in the female SOD3−/−. Furthermore, oxidative stress in post-ischemic kidneys after 1 hour of reperfusion was greater in male than in female mice (3-NT), and even further increased in the male SOD3−/− mice (8-OHdG), suggesting that differences in oxidative stress likely contributed to the observed RBF responses.
Subsequently, we assessed oxidative stress and renal injury in the post-ischemic kidneys after 24 hours of reperfusion. Oxidative stress as determined by 3-NT staining was increased in male and female SOD3−/− mice compared to WT controls, which was associated with increased renal cast formation. Thus, SOD3 deletion leads to greater oxidative stress and renal injury after renal ischemia. Interestingly, females are better able to compensate for the loss of SOD3 in the acute setting of renal ischemia, but this does not translate into less renal injury than that observed in males with SOD3 deletion. Further studies are needed to fully explain this, but the specific cellular location of the three SOD isoforms, SOD3 being extracellular, SOD1 cytoplasmic and SOD2 mitochondrial, may account for this. Even though total SOD activity is preserved in female SOD3−/− mice, there must be a relative lack of SOD activity in the extracellular compartment, and this may be particularly relevant for the delayed injury after renal ischemia.
Several previous studies have demonstrated that administration of either SOD1 or SOD3 ameliorate renal I/R injury2–3, 26–27, but few have provided information on the role of the endogenous SOD isoforms. In keeping with our present study, SOD1-deficient mice demonstrate increased renal staining for 8-OHdG under basal conditions and 24 hours after an ischemic episode28. In conjunction, a greater deterioration of renal function and renal histology was found, but these investigators used a uremia model since mice were nephrectomized on the contralateral side two weeks prior to ischemia. SOD1−/− mice were not able to compensate for loss of SOD1, but there was no mention of whether male or female mice were used. Thus, potential sex differences cannot be excluded. Rubinstein and colleagues reported on the effects of post-ischemic hyperbaric oxygen (HBO) therapy on oxidative stress, renal endothelial function and injury in a rat model of unilateral ischemia29. HBO attenuated the decline of GFR, decreased intrarenal oxidative stress and improved endothelium-dependent vasodilation in the renal vasculature. Most interestingly, these improvements were associated with an increased renal expression of SOD1 in animals treated with HBO. In another model of bilateral ischemia, male mice were protected from I/R injury and oxidative stress after orchidectomy, which was associated with post-ischemic upregulation of SOD2 expression and activity22. These data suggest that testosterone suppresses the induction of SOD2 after ischemia and that SOD2 can also protect against renal I/R injury. Future experiments with tissue-specific targeting of the SOD isoforms, e.g. by using an adenovirus-driven approach30, are expected to provide us with more detailed information on the role of the SOD isoforms in specific tissues, including the kidney.
In conclusion, the current study demonstrates that endogenous SOD3 protects the kidney from renal I/R injury and functions to maintain RBF after ischemia. Although one human trial has studied the effects of recombinant SOD in the context of renal transplantation and has demonstrated no benefit for renal outcome31, the available experimental data suggest that SOD should not be refuted as potential treatment option yet. Furthermore, we found important sex differences, specifically that females with SOD3 deletion, but not males, were able to maintain RBF after ischemia, which was associated with preserved total SOD activity. This demonstrates substantial sex differences in the molecular mechanisms as well as in functional aspects of renal I/R injury. These sex differences should be further studied in order to increase our overall understanding of the mechanisms of renal I/R, which could ultimately lead to identification of new therapeutic targets.
Methods
Renal ischemia/reperfusion model
Male and female C57Blk/6 mice were purchased from Jackson Laboratories (Bar Habor, Maine). To study the effects of SOD3 deletion, SOD3−/− mice on a C57Blk/6 background were used, as described previously17. Mice were studied between 8 and 12 weeks of age, housed in our animal facility with a 12:12 hour light-dark schedule and fed a regular rodent diet containing 0.4% sodium (Harlan Laboratories, Indianapolis, IN). All experiments were approved by the Medical College of Georgia Institutional Animal Care and Use Committee and in accordance with the NIH Guide on the Care and Use of Laboratory Animals.
Mice were anesthetized by continuous inhalation of isofluorane 1% and placed on a servo-controlled surgical heating table. To prevent fluid loss, the right jugular vein was accessed with a polyethylene catheter (PE-10) for constant infusion of 7.5 µl/min of sodium chloride (0.9%) with 1% bovine serum albumin. The left carotid artery was cannulated with a similar catheter connected to a pressure transducer for direct measurement of blood pressure (BP). Subsequently, the mouse was moved into a prone position, the right kidney was accessed, and the renal artery isolated from the renal vein. Renal blood flow (RBF) was then continuously measured via a 0.5V Transonic renal flow probe and a TS420 perivascular flowmeter (Transonic Systems Inc., Ithaca, NY). BP and RBF were analyzed with a PowerLab system (ADInstruments, Boston, MA).
After the surgical procedures and stabilization of hemodynamic parameters, baseline values were determined for an additional 20-minute period. The renal artery was then occluded proximal to the flow probe with a miniature clamp (Fine Science Instruments, Foster City, CA) and zero flow was confirmed in all animals. After 60 minutes of renal ischemia, the clamp was removed to allow for reperfusion of the kidney. After another 60 minutes of reperfusion, the animals were overdosed with isoflurane and blood was obtained through cardiac puncture for measurement of creatinine levels by the Jaffe reaction. Kidneys were removed, weighed, decapsulated and snap-frozen in liquid nitrogen for immunoblotting and assessment of SOD activities. In additional animals, kidneys were immersed in 10% formalin for histological analyses.
Immunoblotting
Renal cortex samples were homogenized and Western blotting was performed as previously described, with minor modifications32. Briefly, following transfer of protein onto PVDF, membranes were blocked in 5% milk in TBS. Two-color immunoblots were performed using polyclonal primary antibodies to SOD1, SOD2 (Stressgen, Victoria, British Columbia, Canada) or SOD3 (provided by Dr. Fukai,33). Specific bands were detected using the Odyssey Infrared Imager (LI-COR Biosciences, Lincoln, NE); AlexaFluor 680 was used for the detection of anti-SOD antibody (Molecular Probes, Eugene, OR) and IRDye800 (Rockland, Gilbertsville, PA) was used for the detection of anti-actin antibody. Actin was used to verify equal protein loading and all densitometric results are reported normalized to actin. Protein concentrations were determined by standard Bradford assay (Bio-Rad, Hercules, CA) using bovine serum albumin as the standard.
SOD activity assays
Renal cortex samples were homogenized and centrifuged at 1,500 g for 5 minutes. Supernatants were diluted (1:200) and 1 µg of protein was assayed for SOD activity (Cayman Chemical, Ann Arbor, MI) according to manufacturer’s instructions. Cyanide (3 mmol/L) was used to distinguish between the cyanide-sensitive isoforms SOD1 and SOD3 and the cyanide-resistant isoform SOD2.
Basal superoxide production and NADPH oxidase activity
Superoxide detection experiments were conducted in 96-well microplates (OptiPlate-96, Perkin-Elmer); 10 to 25 µg of renal cortex homogenate or PSS was added to sample and background wells, respectively, and incubated for 30 minutes at 37°C. Basal superoxide production was measured by lucigenin chemiluminescence (5 µmol/L). NADPH oxidase activity was similarly measured in the presence of 100 µmol/L NADPH (Alexis Biochemicals). After a 30-minute dark-adapt period, plates were counted on a TopCount Microplate Scintillation and Luminescence Counter (Perkin Elmer) set to single-photon counting mode. Superoxide production was expressed as cpm/µg protein. Superoxide detection was abolished with inclusion of tempol (10 mmol/L).
Immunhistochemical analysis
Kidneys were immersed in 10% neutral-buffered formalin overnight at room temperature, transferred to 70% ethanol for 24 h, and paraffin embedded. The kidneys were sectioned at a thickness of 4 µm onto Superfrost plus slides and processed as described previously34. To assess renal injury, kidney sections were stained using a periodic acid-Schiff (PAS) stain kit according to standard methods. The stained sections were viewed with an Olympus B×40 microscope (Olympus America, Melville, NY) on bright-field setting fitted with a digital camera (Olympus DP12; Olympus America). For semiquantitative evaluation, histologic sections were examined for tubular necrosis, interstitial infiltration by multi-nucleated cells and tubular casts. The severity of changes was graded with an arbitrary score of 0 to 4, where 0 was nil, 1 mild, 2 moderate, 3 severe and 4 very severe. To assess oxidative stress, slides were incubated in the absence or presence of primary antibodies to 8-hydroxy-2'deoxyguanosine (8-OHdG; Oxis International, Portland, OR) or 3-nitrotyrosine (3-NT, Upstate Biotechnology) in humidity chambers for 1 h or 30 min, respectively, at room temperature or, for the assessment of infiltration of by macrophages and T-cells, in the absence or presence of primary antibodies to F4/80 (Serotec, Canada) or CD-3 (Santa Cruz Biotechnology, CA) in humidity chambers overnight at 4°C, followed by incubation with peroxidase-conjugated mouse-on-mouse HRP polymer (Biocare) or goat anti-rat IgG (Serotec) for 30 min at room temperature, respectively. For quantification of macrophage and T-cell infiltration, F4/80 and CD-3 positive cells were counted in 10 cortical and 10 medullary 200 × 200-mm sections of kidney cortex and medulla (magnification ×40) and the sum of 10 sections is presented. All assessments were made by an investigator blinded to the specifics of each mouse section.
Statistical analyses
Data analysis was performed using GraphPad Prism4 software. Student` s unpaired t-test were used to compare data between different genotypes and genders, whereas paired t-tests were used for comparisons within the same group of animals. Comparisons between multiple groups were performed by 2-way ANOVA tests followed by Student-Newman-Keuls tests where appropriate. A P-value <0.05 was considered statistically significant. Data are expressed as mean ± standard error.
Supplementary Material
Acknowledgements
We acknowledge the expert technical assistance of Melissa Durley, Carolyn Rhoden, Heather Walker, Janet Hobbs and Amy Dukes. MPS was supported by a fellowship and a research grant from the Deutsche Forschungsgemeinschaft (SCHN 769 and SFB TP B5). Funding support from NIH (JSP: HL60653 and DMP: HL64776) is greatly appreciated.
Footnotes
Disclosure
We hereby declare that no conflict of interest exists.
References
- 1.Linas SL, Whittenburg D, Parsons PE, et al. Ischemia increases neutrophil retention and worsens acute renal failure: role of oxygen metabolites and ICAM 1. Kidney international. 1995;48:1584–1591. doi: 10.1038/ki.1995.451. [DOI] [PubMed] [Google Scholar]
- 2.Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. The Journal of clinical investigation. 1984;74:1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noiri E, Nakao A, Uchida K, et al. Oxidative and nitrosative stress in acute renal ischemia. American journal of physiology. 2001;281:F948–F957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 4.Greene EL, Paller MS. Xanthine oxidase produces O2-. in posthypoxic injury of renal epithelial cells. The American journal of physiology. 1992;263:F251–F255. doi: 10.1152/ajprenal.1992.263.2.F251. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Flecha B, Boveris A. Mitochondrial sites of hydrogen peroxide production in reperfused rat kidney cortex. Biochimica et biophysica acta. 1995;1243:361–366. doi: 10.1016/0304-4165(94)00160-y. [DOI] [PubMed] [Google Scholar]
- 6.Perianayagam MC, Liangos O, Kolyada AY, et al. NADPH oxidase p22phox and catalase gene variants are associated with biomarkers of oxidative stress and adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18:255–263. doi: 10.1681/ASN.2006070806. [DOI] [PubMed] [Google Scholar]
- 7.Leaf A. Cell swelling. A factor in ischemic tissue injury. Circulation. 1973;48:455–458. doi: 10.1161/01.cir.48.3.455. [DOI] [PubMed] [Google Scholar]
- 8.Lieberthal W, Wolf EF, Rennke HG, et al. Renal ischemia and reperfusion impair endothelium-dependent vascular relaxation. The American journal of physiology. 1989;256:F894–F900. doi: 10.1152/ajprenal.1989.256.5.F894. [DOI] [PubMed] [Google Scholar]
- 9.Brodsky SV, Yamamoto T, Tada T, et al. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. American journal of physiology. 2002;282:F1140–F1149. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- 10.Guan Z, Gobe G, Willgoss D, et al. Renal endothelial dysfunction and impaired autoregulation after ischemia-reperfusion injury result from excess nitric oxide. American journal of physiology. 2006;291:F619–F628. doi: 10.1152/ajprenal.00302.2005. [DOI] [PubMed] [Google Scholar]
- 11.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney international. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 12.Conger JD, Robinette JB, Hammond WS. Differences in vascular reactivity in models of ischemic acute renal failure. Kidney international. 1991;39:1087–1097. doi: 10.1038/ki.1991.138. [DOI] [PubMed] [Google Scholar]
- 13.Gellai M, Jugus M, Fletcher T, et al. Reversal of postischemic acute renal failure with a selective endothelinA receptor antagonist in the rat. The Journal of clinical investigation. 1994;93:900–906. doi: 10.1172/JCI117046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharfuddin AA, Sandoval RM, Berg DT, et al. Soluble Thrombomodulin Protects Ischemic Kidneys. J Am Soc Nephrol. 2009 doi: 10.1681/ASN.2008060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ookawara T, Imazeki N, Matsubara O, et al. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am J Physiol. 1998;275:C840–C847. doi: 10.1152/ajpcell.1998.275.3.C840. [DOI] [PubMed] [Google Scholar]
- 16.Stralin P, Karlsson K, Johansson BO, et al. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arteriosclerosis, thrombosis, and vascular biology. 1995;15:2032–2036. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- 17.Gongora MC, Qin Z, Laude K, et al. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 18.Kim HW, Lin A, Guldberg RE, et al. Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circulation research. 2007;101:409–419. doi: 10.1161/CIRCRESAHA.107.153791. [DOI] [PubMed] [Google Scholar]
- 19.Hutchens MP, Dunlap J, Hurn PD, et al. Renal ischemia: does sex matter? Anesthesia and analgesia. 2008;107:239–249. doi: 10.1213/ane.0b013e318178ca42. [DOI] [PubMed] [Google Scholar]
- 20.Wei Q, Wang MH, Dong Z. Differential gender differences in ischemic and nephrotoxic acute renal failure. American journal of nephrology. 2005;25:491–499. doi: 10.1159/000088171. [DOI] [PubMed] [Google Scholar]
- 21.Park KM, Kim JI, Ahn Y, et al. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. The Journal of biological chemistry. 2004;279:52282–52292. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Kil IS, Seok YM, et al. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. The Journal of biological chemistry. 2006;281:20349–20356. doi: 10.1074/jbc.M512740200. [DOI] [PubMed] [Google Scholar]
- 23.Kher A, Meldrum KK, Wang M, et al. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovascular research. 2005;67:594–603. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Singh I, Gulati S, Orak JK, et al. Expression of antioxidant enzymes in rat kidney during ischemia-reperfusion injury. Molecular and cellular biochemistry. 1993;125:97–104. doi: 10.1007/BF00936438. [DOI] [PubMed] [Google Scholar]
- 25.Dobashi K, Ghosh B, Orak JK, et al. Kidney ischemia-reperfusion: modulation of antioxidant defenses. Molecular and cellular biochemistry. 2000;205:1–11. doi: 10.1023/a:1007047505107. [DOI] [PubMed] [Google Scholar]
- 26.Yin M, Wheeler MD, Connor HD, et al. Cu/Zn-superoxide dismutase gene attenuates ischemia-reperfusion injury in the rat kidney. J Am Soc Nephrol. 2001;12:2691–2700. doi: 10.1681/ASN.V12122691. [DOI] [PubMed] [Google Scholar]
- 27.Mihara K, Oka Y, Sawai K, et al. Improvement of therapeutic effect of human recombinant superoxide dismutase on ischemic acute renal failure in the rat via cationization and conjugation with polyethylene glycol. Journal of drug targeting. 1994;2:317–321. doi: 10.3109/10611869409015912. [DOI] [PubMed] [Google Scholar]
- 28.Yamanobe T, Okada F, Iuchi Y, et al. Deterioration of ischemia/reperfusion-induced acute renal failure in SOD1-deficient mice. Free radical research. 2007;41:200–207. doi: 10.1080/10715760601038791. [DOI] [PubMed] [Google Scholar]
- 29.Rubinstein I, Abassi Z, Milman F, et al. Hyperbaric oxygen treatment improves GFR in rats with ischaemia/reperfusion renal injury: a possible role for the antioxidant/oxidant balance in the ischaemic kidney. Nephrol Dial Transplant. 2009;24:428–436. doi: 10.1093/ndt/gfn511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lob HE, Marvar PJ, Guzik TJ, et al. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension. 2010;55:277–283. doi: 10.1161/HYPERTENSIONAHA.109.142646. 276p following 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollak R, Andrisevic JH, Maddux MS, et al. A randomized double-blind trial of the use of human recombinant superoxide dismutase in renal transplantation. Transplantation. 1993;55:57–60. doi: 10.1097/00007890-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan JC, Pollock DM, Pollock JS. Altered nitric oxide synthase 3 distribution in mesenteric arteries of hypertensive rats. Hypertension. 2002;39:597–602. doi: 10.1161/hy0202.103286. [DOI] [PubMed] [Google Scholar]
- 33.Fukai T, Galis ZS, Meng XP, et al. Vascular expression of extracellular superoxide dismutase in atherosclerosis. The Journal of clinical investigation. 1998;101:2101–2111. doi: 10.1172/JCI2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishii N, Patel KP, Lane PH, et al. Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J Am Soc Nephrol. 2001;12:1630–1639. doi: 10.1681/ASN.V1281630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.