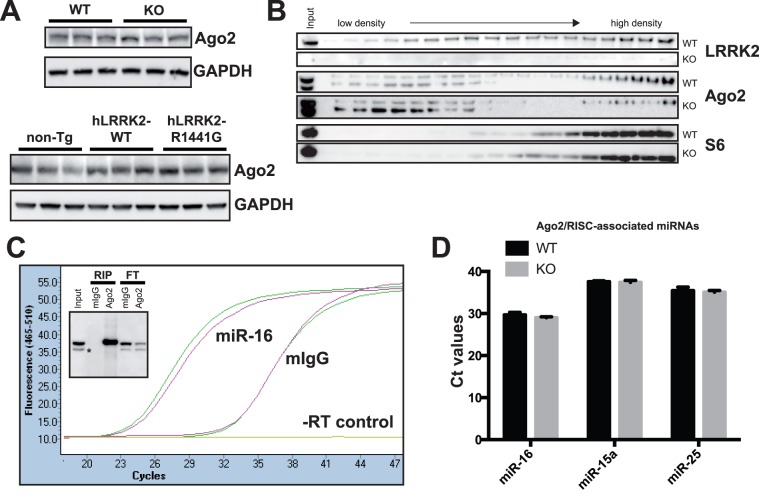
Figure 4. Biochemical and functional analysis of Ago2 in LRRK2 mice.
(A) Representative western blot analysis showing that Ago2 protein levels are not affected in the presence or absence of LRRK2 (top panel). Overexpression of human LRRK2-WT or harbouring the human PD mutation R1441G has no effect on Ago2 levels either, when compared to non-transgenic littermates (bottom panel). (B) Polysomes fractionation of LRRK2-deficient mouse brain, compared to wild-type brain. No change in Ago2 or the ribosomal protein S6 distribution was observed by the absence of LRRK2. (C) RIP-Ago2 assay of wild-type mouse brain. A representative amplification curve of miR-16 by real-time qRT-PCR shows a significant enrichment (∼500 fold) of this miRNA pulled down by RIP-Ago2. The insert demonstrates the efficiency of the immunoprecipitation (Ago2 vs. mouse IgGs). The flow-through (FT) shows the concomitant decrease of Ago2 in the post-RIP lysate. The “*” sign is Radixin, a well known non-specific protein when using the Ago2 (2A8) antibody [47]. Of note, Radixin is not immunoprecipitated by Ago2 (2A8). (D) Histogram of RIP miRNA pulled down in the presence or absence of LRRK2. In each case (n = 3), there is no difference in Ct values (qRT-PCR). LRRK2 deficiency does not affect RIP-Ago2 RNAs or miRNAs immunoprecipitation. Standard error of the mean (SEM) is shown.