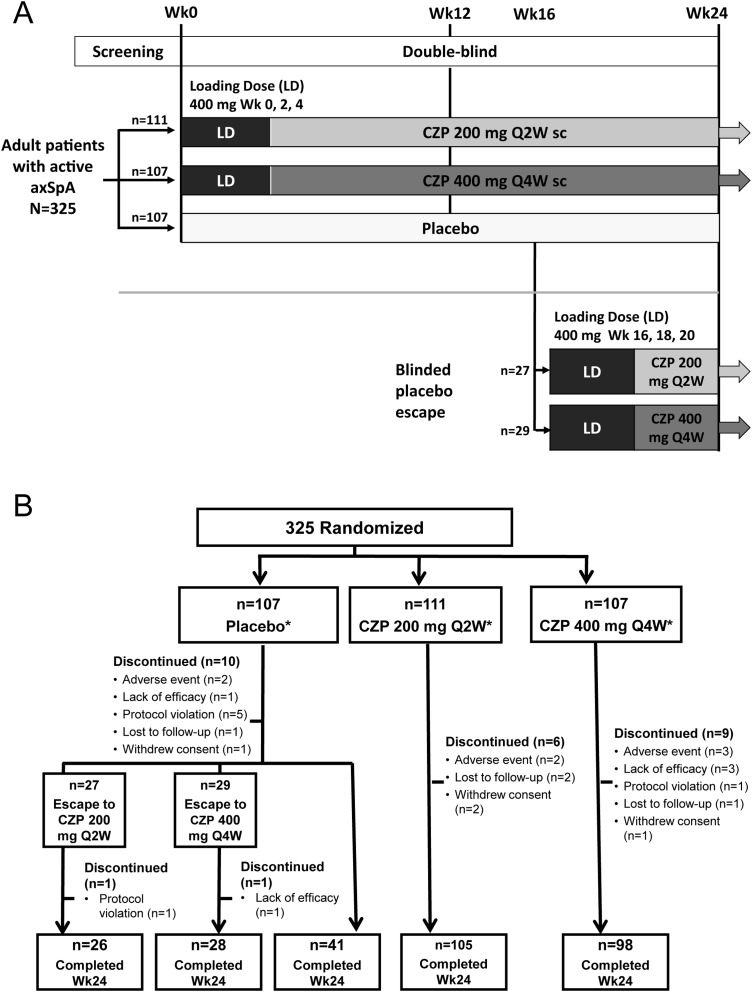
Figure 1.
Study design and patient disposition. A. RAPID-axSpA trial design to week 24 of an ongoing 204-week trial. B. Disposition of patients in the overall axSpA population (RS). axSpA, axial spondyloarthritis; CZP, certolizumab pegol; LD, loading dose; Q2W, every 2 weeks; Q4W, every 4 weeks; sc, subcutaneous. *All patients received the allocated treatment. Discontinuations for placebo patients are detailed for time periods prior to and after escape at week 16.