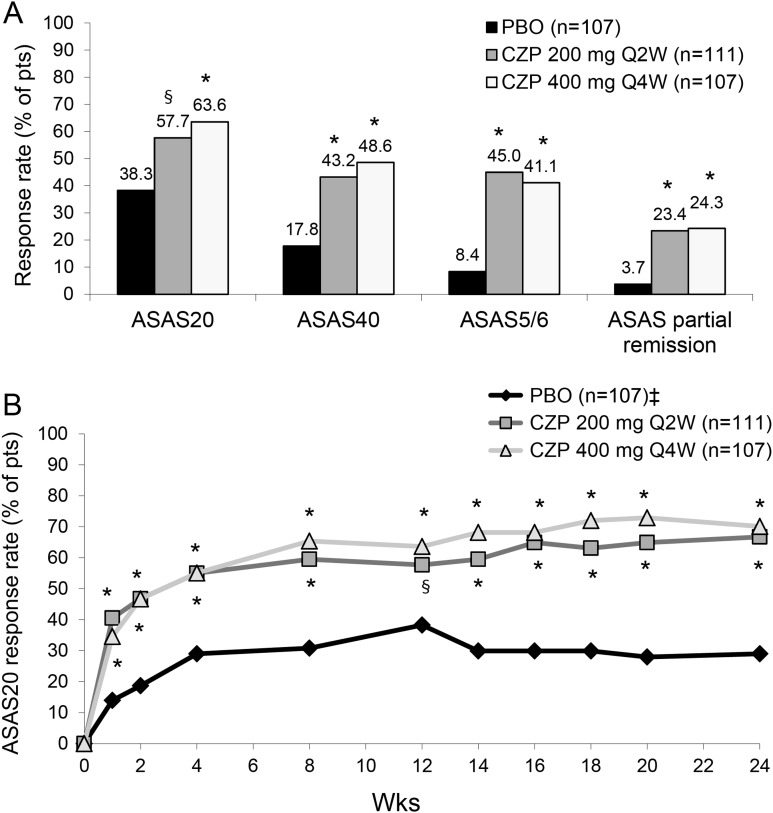
Figure 2.
ASAS response rates in axSpA patients at week 12 (A) and up to week 24 (B). A. ASAS20, ASAS40, ASAS5/6 and ASAS partial remission response rates at week 12. * p<0.001; § p=0.004 CZP versus PBO (2-sided Wald asymptotic test). B. ASAS20 response rate in the three treatment groups from week 0 to week 24. * p<0.001; § p=0.004 CZP versus PBO (2-sided Wald asymptotic test). ‡ Placebo patients escaping at week 16 (n=56) were considered non-responders at weeks 16–24. Data corresponds to the RS (non-responder imputation). PBO, placebo; CZP, certolizumab pegol; Q2W, every 2 weeks; Q4W, every 4 weeks; ASAS20, assessment of Axial SpondyloArthritis international Society 20% response criteria; ASAS40, assessment of Axial SpondyloArthritis international Society 40% response criteria; ASAS5/6: assessment of Axial SpondyloArthritis international Society at least 20% improvement in 5 of 6 domains (including spinal mobility and c-reactive protein).