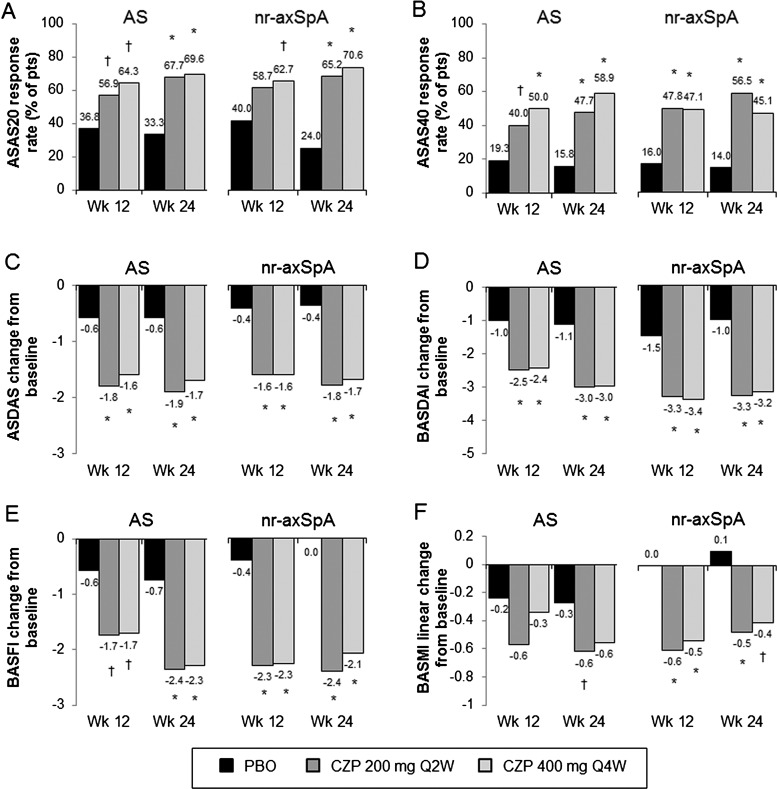
Figure 4.
Efficacy in AS and nr-axSpA subpopulations at weeks 12 and 24. Responses for the three treatment groups (placebo (PBO), CZP 200 mg Q2W or CZP 400 mg Q4W) at weeks 12 and 24 in: A, ASAS20; B, ASAS40 and C, ASDAS. Change from baseline at weeks 12 and 24 in: D, BASDAI; E, BASFI and F, BASMI linear. All response rates are presented as % of patients. * p<0.001; † p<0.05 CZP versus PBO (2-sided Wald asymptotic test). Escaping PBO patients were considered non-responders week 24. N values for the AS subpopulation are 57, 65 and 56 for the placebo, CZP 200 mg Q2W and CZP 400 mg Q4W. AS, ankylosing spondylitis; ASAS20, assessment of Axial SpondyloArthritis international Society 20% response criteria; ASAS40, assessment of Axial SpondyloArthritis international Society 40% response criteria; ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index linear; CZP, certolizumab pegol.