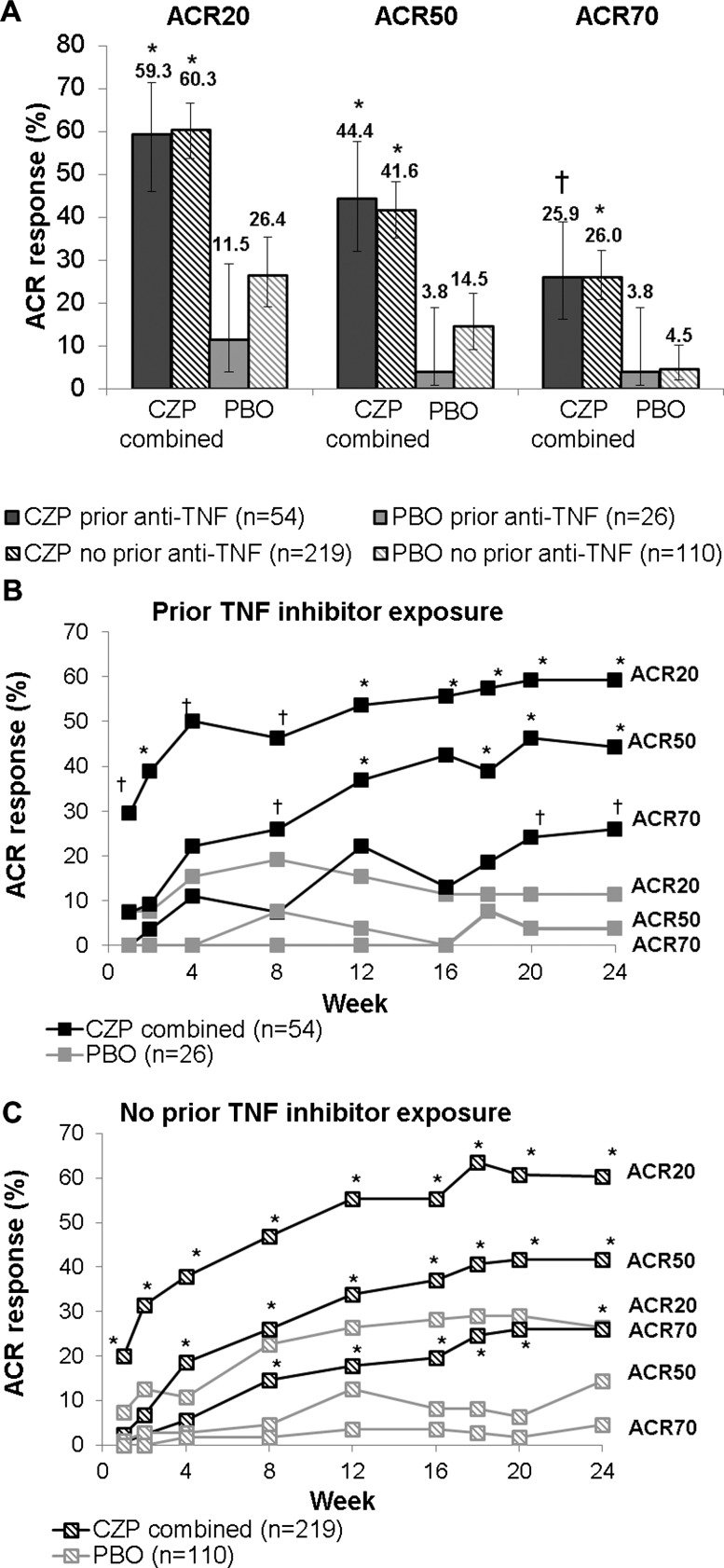
Figure 3.
Effect of CZP in patients with and without prior TNF inhibitor exposure at week 24 in terms of ACR response and the kinetics of ACR response. CZP, Certolizumab pegol; Q2W, every 2 weeks; Q4W, every 4 weeks; PBO, placebo; TNF, tumour necrosis factor. (A) Percentages of patients with and without prior TNF inhibitor exposure achieving a response according to the American College of Rheumatology Criteria for 20% improvement (ACR20), 50% improvement (ACR50), and 70% improvement (ACR70) at week 24, by treatment group. (B) Percentages of patients with prior TNF inhibitor exposure achieving an ACR20, ACR50, and ACR70 response over time, by treatment group. (C) Percentages of patients without prior TNF inhibitor exposure achieving an ACR20, ACR50 and ACR70 response over time, by treatment group; *p<0.001; †p<0.05 versus placebo.