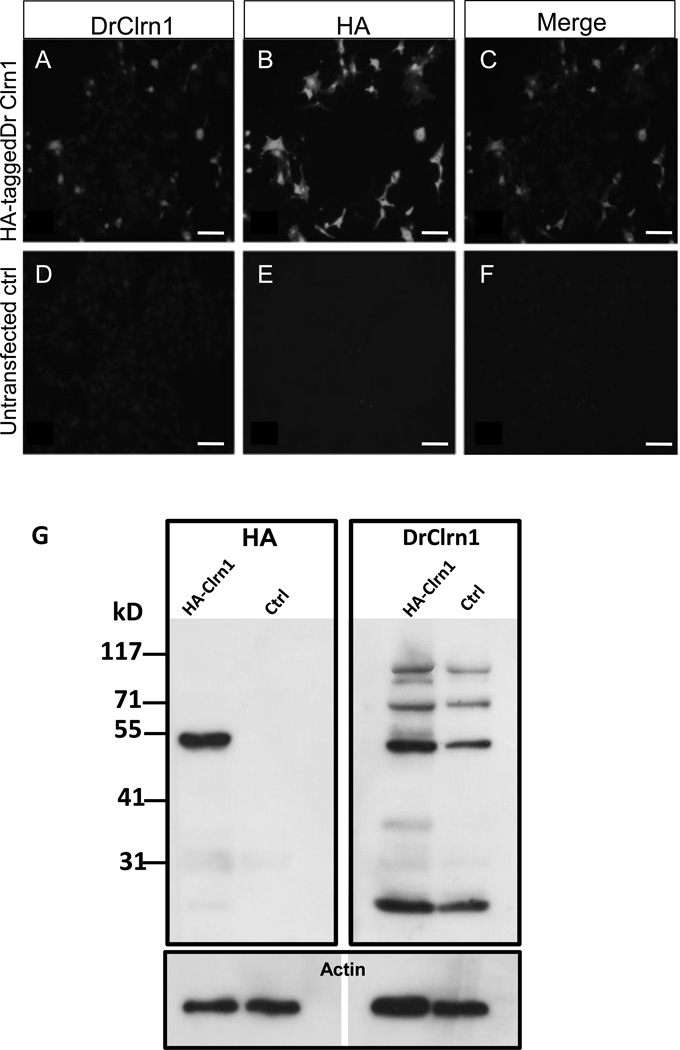
Fig. 3.
DrClrn1 antibody specifically recognizes Clarin-1 protein. Full-length zebrafish clrn1 was cloned into an HA-tag containing vector and transfected into BHK cells. Transfected cells were positively identified when incubated with DrClrn1 (A) or HA (B) antibodies. Clarin-1 and HA merged image shown in C. No signal was detected with either antibody in untransfected cell cultures (D–F). (G) Western blot of protein extracts from whole 30 hpf Tg(hsp70l:Gal4) larvae using an anti HA-antibody (left blot) or DrClrn1 antibody (right). Protein extracts from embryos injected with a Clrn1-HA construct under the control of a UAS promoter and from uninjected Tg(hsp70l:Gal4) embryos were obtained 6 hours after heat shock treatment. The anti-HA recognizes an approximately 52 kD band in the injected sample, which is absent from the control lane, and faintly labels a band at 25 kD. An additional faint band is noted at approximately 31 kD on the membrane incubated with anti-HA, but as this is also detectable in the uninjected control lane, it appears to be nonspecific. Multiple bands are present in extracts from injected and uninjected embryos incubated with DrClrn1. The band migrating at approximately 25 kD is consistent with the predicted size of the Clarin-1 protein. The approximately 52 kD band is consistent with the presence of Clarin-1 homodimers, and bands of higher weights are consistent with multimers. Membranes were subsequently probed with anti-pan actin as a loading control (lower panel). Scale bars: 50 µm.