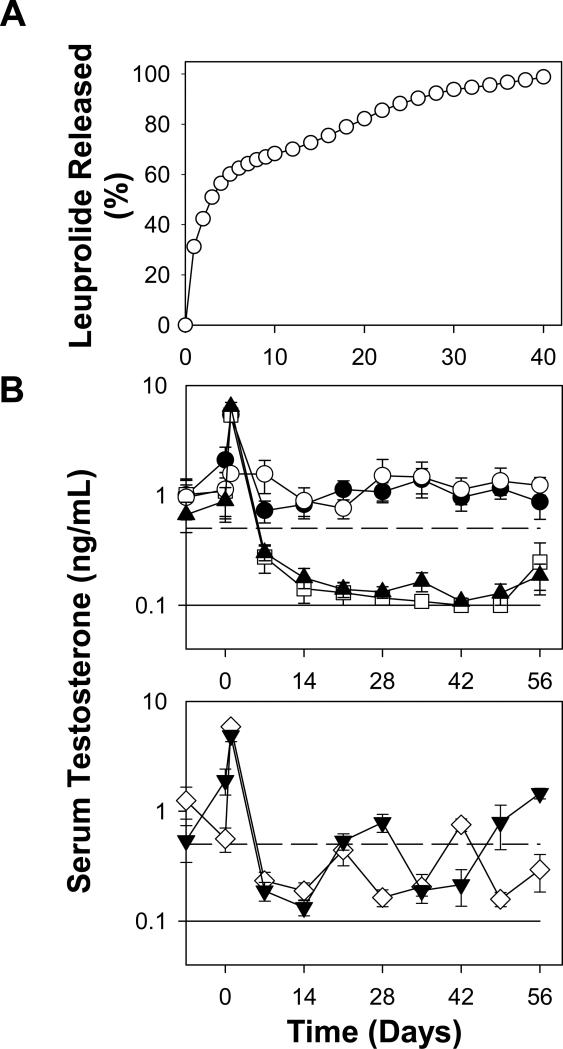
Fig. 4.
Long-term in vitro leuprolide release behavior (A) and in vivo testosterone suppression ability (B) of leuprolide-absorbed PLGA-COOH formulation. Studies were conducted in PBST + 0.02% sodium azide at 37 °C (A) or male Sprague-Dawley rats (B). Animals were injected subcutaneously with soluble leuprolide at day 0 (●), leuprolide-PLGA particles with various dosing intervals (2 week: days 0, 14, 28, and 42 (▲), 3 week: days 21 and 42 (◇), and 4 week: days 0 and 28 (▼)), and blank PLGA particles (○) along with commercial 1-month Lupron Depot® at days 0 and 28 (□). Leuprolide dose was 100 μg/kg/day. Solid and dashed line respectively represents lower testosterone detection limit (0.1 ng/mL) and castration level (0.5 ng/mL). Symbols represent mean ± SEM [n = 3 (A) or 6 (B)] with error bars not visible when smaller than symbols.