Figure 3.
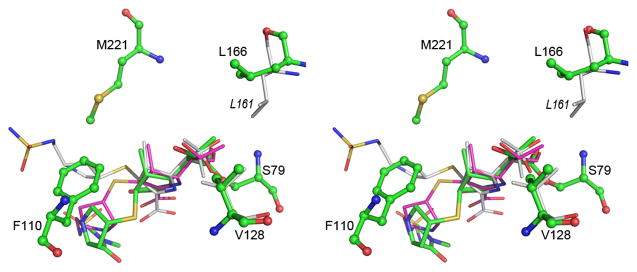
Stereoview of the superposition of the acyl-enzyme intermediate in OXA-23 (green), OXA-24 (magenta) and OXA-1 (white). The two hydrophobic residues which interact with the 6a-hydroxyethyl moiety of the intermediate are shown at upper right (Leu166 for OXA-23 and Leu161 for OXA-1) and lower right (Val128 for OXA-23). The difference in conformation of the leucine residue can be seen. The residues which constitute the tunnel-like structure in the OXA enzymes are indicated for OXA-23 (Phe110 and Met221). See also Figure S3 and Table S1.
