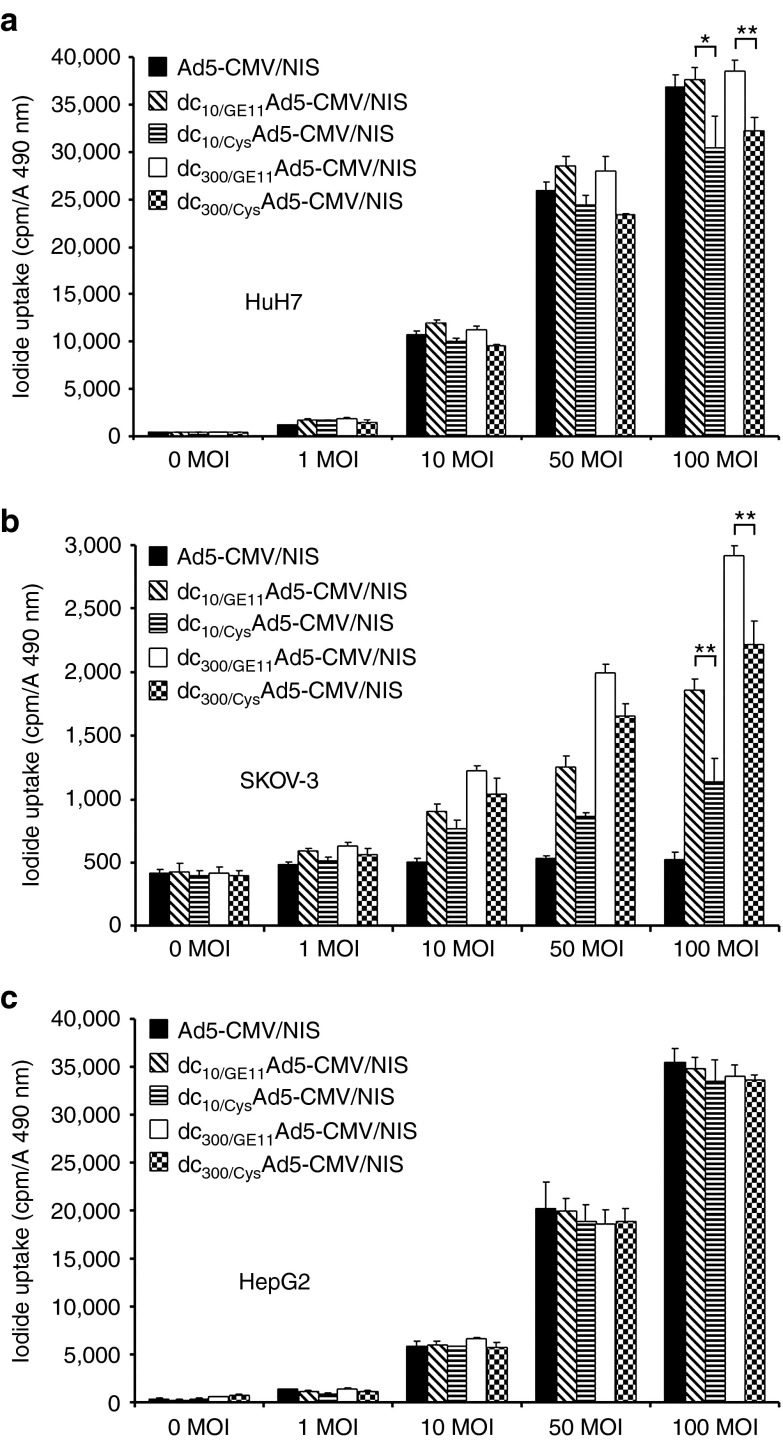
Figure 1.
In vitro iodide uptake studies of EGFR-targeted adenovirus. In vitro transduction experiments with uncoated Ad5-CMV/NIS showed dose-dependent transduction efficiency in CAR-positive cells (HuH7, HepG2), which was fully retained after EGFR-targeted coating of the adenovirus with increasing amounts of dendrimer (a, c). The CAR-negative cell line SKOV-3 showed no iodide accumulation above background level, even when incubated with high MOI of the uncoated Ad5-CMV/NIS but adenoviral coating with increasing amounts of EGFR-targeted dendrimer caused a significant increase in perchlorate-sensitive iodide uptake activity (b; **P < 0.01). Replacement of the targeting ligand GE11 by a cysteine residue (Cys) lowered transduction efficiency in EGFR-positive HuH7 and SKOV-3 cells (a: *P < 0.05, **P < 0.01; b: **P < 0.01) whereas transfection efficiency in the low EGFR expressing HepG2 cells remained unchanged (c). CAR, coxsackie-adenovirus receptor; EGFR, epidermal growth factor receptor; MOI, multiplicity of infection; NIS, sodium iodide symporter.