Abstract
In rats, considerable differences in the social consequences of acute ethanol are seen across ontogeny, with adolescents being more sensitive to low dose ethanol-induced social facilitation and less sensitive to the social inhibition evident at higher ethanol doses relative to adults. Stressor exposure induces social anxiety-like behavior, indexed via decreases in social preference, and alters responsiveness to the social consequences of acute ethanol by enhancing ethanol-associated social facilitation and anxiolysis regardless of age. Given that substantial ontogenetic differences in the social consequences of ethanol are evident even within the adolescent period, the present study was designed to investigate whether similar stress-associated alterations in social behavior and ethanol responsiveness are evident in early and late adolescents. Juvenile-early adolescent [postnatal days (P) 24-28] and mid-late adolescent (P38-42) male and female Sprague-Dawley rats were repeatedly restrained (90 min/day) for 5 days, followed by examination of ethanol-induced (0, 0.25, 0.5, or 1.0 g/kg) alterations in social behaviors on the last day. Responsiveness to restraint stress in terms of both stress-induced behavioral alterations and stress-associated changes in sensitivity to the social consequences of acute ethanol challenge differed drastically at the two ages. Repeated restraint increased anxiety-like behavior in a social context in older adolescents, whereas the previously stressed young adolescent males showed substantial increases in play fighting – an effect of stress not evident in P28 females or P42 adolescents of either sex. Unexpectedly, repeated restraint eliminated sensitivity to ethanol-induced social facilitation in P28 adolescent males and made their female counterparts less sensitive to this effect. In contrast, previously stressed late adolescents became sensitive to the socially facilitating and anxiolytic effects of acute ethanol.
Keywords: adolescent, anxiety, ethanol, rat, social behavior, social interaction test, restraint, repeated stress
1. Introduction
In humans, adolescence refers to a transitional period between youth and maturity occurring predominantly during the second decade of life, with females generally showing more rapid maturation than males (Petersen et al., 1996). This gradual transformation from immaturity/dependence to maturity/independence is a developmental phase that can be identified across different mammalian species (Spear, 2010), with adolescent animals often differing notably from those younger or older in the way they respond to stimuli in their environment (Doremus-Fitzwater et al., 2010; Spear, 2000). In rats, a conservative age range during which adolescent-characteristic behavioral and neural features are evident in males and females is the range between P28 and P42 (Spear, 2000), although this timing may vary depending on the growth rate of animals (Kennedy & Mitra, 1963) and the maturational index used. Whereas the onset of adolescence is generally accepted to be between postnatal days 28-32 (P28-32), offset times have been suggested to vary between P38-50 or even later (Odell, 1990; Schneider, 2013). Thus, the broad adolescent age range has recently been subdivided into three developmental phases, namely early adolescence (P28-35), mid adolescence (P35-42), and late adolescence/emerging adulthood (between approximately P42 and P55) (e.g., Vetter-O'Hagen and Spear, 2012).
In humans, adolescence as a developmental period is characterized by high levels of alcohol use, with approximately 5.1% of 8th graders, 15.6% of 10th graders, and 23.7% of high school seniors in the United States reporting a binge pattern of drinking (5+ drinks in a row) in the last two weeks (Johnston et al., 2013), and even more elevated rates of binge drinking reported among adolescents in many European countries (Ahlstrom & Osterberg, 2005). High levels of ethanol consumption are not restricted to human adolescents but may be seen in adolescent rodents as well, with adolescent rats ingesting more ethanol on a g/kg basis than adults (Broadwater et al., 2011; Brunell and Spear, 2005; Doremus et al., 2005; Vetter et al., 2007; Vetter-O'Hagen et al., 2009). These high levels of ethanol intake during adolescence may be related to adolescent-typical insensitivities to a number of adverse ethanol effects that serve to moderate drinking under normal circumstances (Spear and Varlinskaya, 2010). For instance, adolescent rats are less sensitive than adults to ethanol-induced social inhibition and anxiogenesis (Varlinskaya and Spear, 2002), sedation (Draski et al., 2001; Moy et al., 1998; Silveri & Spear, 1998), motor impairment (Ramirez and Spear, 2010; White et al., 2002), and taste aversion (Anderson et al., 2010; Schramm-Sapyta et al., 2010; Vetter-O'Hagen et al., 2009). However, adolescent rats are conversely extremely sensitive to ethanol-induced social facilitation, demonstrating pronounced social activation following low doses of ethanol that is not normally evident in adult rats (Trezza et al., 2009; Varlinskaya and Spear, 2002). Considerable ontogenetic differences in the social consequences of acute ethanol are seen even within the adolescent period, with early adolescence being a time when adolescent-typical sensitivities to ethanol are particularly pronounced. For instance, early adolescent rats tested on P28 are more sensitive to low dose ethanol-induced social facilitation and less sensitive to the social inhibition evident at higher ethanol doses than animals tested during late adolescence at P42 (Varlinskaya and Spear, 2004, 2006).
Sensitivity to the social consequences of ethanol can be modified by stress in both mid adolescent and adult rats. Repeated restraint stress exacerbated adolescent-typical responsiveness to the social consequences of acute ethanol challenge in mid adolescent (P35) animals, enhancing adolescent-typical sensitivity to the stimulatory effects of ethanol on play fighting and further attenuating adolescent-characteristic insensitivities to the socially suppressing effects of ethanol (Varlinskaya et al., 2010). Surprisingly, among adults tested at P70, prior repeated restraint induced an adolescent-typical pattern of responsiveness to the social consequences of ethanol, precipitating ethanol-induced facilitation of play fighting and eliminating the social inhibition induced by higher ethanol doses in non-stressed animals. Furthermore, anxiety-like behavioral alterations induced by repeated restraint and indexed via decreases in social preference and social investigation were effectively attenuated by acute ethanol in both mid adolescents and adults, whereas no effects of stress were evident on ethanol-associated inhibition of locomotor activity (observed at 1.0 g/kg ethanol) under social test circumstances regardless of age (Varlinskaya et al., 2010). These findings suggest that repeated stress diminishes age-related differences in sensitivity to the social consequences of ethanol and makes both adolescent and adult animals unusually sensitive to ethanol-associated anxiolysis.
Given that substantial ontogenetic differences in the social consequences of ethanol are evident even within the adolescent period (Varlinskaya and Spear, 2004, 2006), the present study was designed to investigate: (1) whether typical developmental differences in sensitivity to the social consequences of acute ethanol seen between early and late adolescents are diminished or even eliminated by prior exposure to restraint, and (2) whether repeated restraint similarly enhances sensitivity of both younger and older adolescents to the socially anxiolytic effects of ethanol. For animals tested as early adolescents on P28 repeated stressor exposure occurred during juvenile period, whereas late adolescents tested at P42 were exposed to restraint during mid adolescence.
2. Methods
2.1. Subjects
Adolescent Sprague-Dawley male and female rats bred and reared in our colony at Binghamton University were used. A total of 48 litters provided 192 male and female offspring to serve as experimental subjects and 192 to serve as partners. Animals were housed in a temperature-controlled (22°C) vivarium, and maintained on a 12:12 hr light:dark cycle (lights on at 0700 hr) with ad libitum access to food (Purina rat chow) and water. Litters were culled to 10 pups (five males and five females) within 24 hr after birth on P0 and reared until weaning with their mothers in standard plastic maternity cages with pine shavings as bedding material. Rats were weaned on P21 and housed with their same-sex littermates. At all times, rats used in the current study were produced, maintained, and treated in accordance with the guidelines for animal care established by the National Institutes of Health, using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
2.2. Experimental Design
The design was a 2 (age: early, late adolescent) × 2 (sex) × 2 (stress condition: no stress or repeated restraint) × 4 (ethanol dose: 0, 0.25, 0.5, and 1.0 g/kg) factorial, with six experimental animals tested per group. Males and females were tested either on P28 (early adolescents) or on P42 (late adolescents). All animals from a given litter were assigned to the same stress condition. To avoid the possible confounding of litter with the experimental variables (Holson and Pearce, 1992; Zorrilla, 1997), no more than one subject of a given sex from a given litter was assigned to a particular ethanol dose/stress condition, with order of testing counterbalanced across litters.
2.3. Stressor Procedures
Beginning at P24 for animals to be tested as early adolescents or at P38 for those to be tested as late adolescents, rats from the repeated stress group were removed from their home cage between 1000 – 1200 hr and then restrained in an age size-adjusted restraint tube for 90 min in a novel holding cage. Restraint tubes (Braintree Scientific, Braintree, MA) were round slotted Plexiglas cylinders with sliding plugs to allow adjustment of the tube length for each animal's size. Cylinders measured 18.0 × 4.7 cm for the juvenile-early adolescent rats and 20.5 × 7.0 cm for mid-late adolescents (length × diameter). For animals in the stress group, this restraint procedure was repeated each day for 5 days. Animals placed in the control condition were non-manipulated throughout the 5-day stressor phase until the time of ethanol challenge, except for body weighing on P24 or P38.
As in our previous studies (Doremus-Fitzwater et al., 2009; Varlinskaya et al., 2010; Varlinskaya and Spear, 2012), restraint was used as a stressor, since this stressor is primarily psychological in nature and does not induce physical pain or harm to an experimental subject (Herman and Cullinan, 1997; Weinberg et al., 2007).
Percentage body weight gain from day 1 (P24 or P38) to day 5 (P28 or P42) was calculated for males and females from each stress condition using litter as a unit of analysis (i.e., analyzing mean body weights for animals of each sex within each litter, see Holson and Pearce, 1992).
2.4. Ethanol Challenge
Ethanol was injected intraperitoneally (i.p.) as a 12.6% (v/v) solution in saline (0.9%, w/v) at doses of 0, 0.25, 0.5, and 1.0 g/kg. Ethanol challenge dose was varied by altering the volume of the 12.6% ethanol solution to avoid concentration-induced differences in ethanol absorption rate (see Linakis and Cunningham, 1979). Control animals were injected with isotonic saline at a volume equal to that of the highest dose of ethanol administered. All solutions were injected at room temperature. Similar to our previous work (e.g., Varlinskaya & Spear, 2002, 2006, 2012; Varlinskaya et al., 2010), the i.p. route of ethanol administration was employed in this study, given that it produces little variability in blood ethanol levels and has been the most commonly used route of administration in neuropharmacological studies of acute ethanol effects.
2.5. Testing Procedures
Immediately after the 90-min stressor exposure on day 5 (or upon removal from the home cage for non-stressed animals), each subject was injected with one of the four doses of ethanol. Immediately after drug administration, each experimental animal was placed alone into the testing chamber for 30 min. This pretest familiarization was conducted to increase baseline levels of social interaction during testing, hence making potential anxiogenic effects of the repeated stressors easier to observe (Willey et al., 2009). A same age and sex test partner unfamiliar with both the test apparatus and the experimental animal was then placed into the apparatus, and social interactions were recorded for 10 min. Partners were always non-stressed, drug-naive animals that had not been socially isolated prior to testing. Weight differences between test subjects and their partners were minimized as much as possible, with this weight difference not exceeding 5 g for animals at P28 and 10 g at P42, and test subjects always being heavier than their partners. The order of testing was counterbalanced for all experimental conditions.
Testing was conducted in Plexiglas test chambers (30 × 20 × 20 cm) that contained clean pine shavings. The test apparatuses (Binghamton Plate Glass, Binghamton, NY) were divided into two compartments by a clear Plexiglas partition containing an aperture (7 × 5 cm) to allow movement of animals between compartments (Varlinskaya et al., 1999, 2001). Each 10-min social interaction test session was conducted under dim light (15-20 lux) between 1200 and 1600 hr, with a white noise generator used to attenuate extraneous sounds during testing. The behavior of each pair was recorded by a video camera mounted above the apparatus.
2.6. Behavioral Measures
The frequencies of social investigation and play fighting were analyzed from video recordings (Vanderschuren et al., 1997; Varlinskaya and Spear, 2002, 2006, 2008a) by a trained experimenter without knowledge of the experimental condition of any given animal. Social investigation was defined as the sniffing of any part of the body of the partner. Play fighting was scored as the sum of the frequencies of the following behaviors: pouncing or playful nape attack (experimental subject lunges at the partner with its forepaws extended outward); following and chasing (experimental animal rapidly pursues the partner); and pinning (the experimental subject stands over the exposed ventral area of the partner, pressing it against the floor). Play fighting can be distinguished from serious fighting in the laboratory rat by the target of the attack—during play fighting, snout or oral contact is directed towards the partner's nape, whereas during serious fighting the partner's rump is the object of the attack (Pellis and Pellis, 1987). Aggressive behavior (serious fighting) was not analyzed in these experiments, since subjects did not exhibit serious attacks or threats.
Social preference/avoidance was assessed by separately measuring the number of crossovers demonstrated by the experimental subject towards as well as away from the social partner and was indexed by means of a coefficient of preference/avoidance [coefficient (%) = (crossovers to the partner – crossovers away from the partner)/(total number of crosses both to and away from the partner) × 100]. Social preference was defined as positive values of the coefficient, while social avoidance was associated with negative values (Varlinskaya et al., 1999).
The total number of crossovers (movements between compartments through the aperture to and from the social partner) exhibited by each experimental subject was used as an index of locomotor activity in the social context (Varlinskaya et al., 1999).
2.7. Blood Ethanol Determination
For analysis of blood ethanol content (BEC), trunk blood samples were collected immediately after behavioral testing using heparinized tubes. Blood samples were then rapidly frozen and maintained at –80°C. Samples were assessed for BECs via headspace gas chromatography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-μl aliquots were placed in airtight vials. Vials were placed in a HP 7694E Auto-Sampler, which heated each individual vial for 8 min and then extracted and injected a 1.0 ml sample of the gas headspace into the chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software, which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions.
2.8. Data Analyses
Data for each dependent variable (play fighting, social investigation, preference coefficient, and total number of crossovers) were analyzed using separate 2 (age) × 2 (sex) × 2 (stress condition) × 4 (ethanol dose) ANOVAs. Significant age × stress × ethanol dose interactions emerged in these overall ANOVAs for play fighting, social investigation, and preference coefficient (all p < .05), suggesting pronounced age-related differences not only in ethanol sensitivity but in stress responsiveness as well. Therefore, each of the behavioral measures was examined separately at each age using 2 (stress condition) × 2 (sex) × 4 (ethanol dose) ANOVAs. In order to avoid inflating the possibility of type II errors in tests with at least three factors (Carmer and Swanson, 1973), Fisher's planned pair-wise comparison test was used to explore significant effects and interactions. Where significant interactions involving stress condition and ethanol challenge dose were evident, ethanol-induced changes were assessed between ethanol-challenged animals and saline-challenged controls within each stress condition. Fisher planned comparisons were also conducted between non-manipulated and stressed animals challenged with saline within each age group for the assessment of stress effects on each of the social behavioral measures.
3. Results
3.1. Body weight gain (Table 1)
Table 1.
Percent body weight gain from day 1 to day 5 in non-stressed and stressed P28 and P42 rats
| Sex | Body Weight Gain (%) P28 | Body Weight Gain (%) P42 | ||
|---|---|---|---|---|
| No Stress | Stress | No Stress | Stress | |
| Male | 30.6 ± 1.2 | 23.7 ± 1.2* | 17.9 ± 0.4 | 12.6 ± 0.9* |
| Female | 27.6 ± 1.1 | 19.2 ± 1.0* | 14.8 ± 3.9 | 8.0 ± 1.0* |
Asterisks indicate a significant differences from the non-manipulated group, collapsed across sex and age (main effect of stress condition).
A 2 (stress condition) × 2 (age) × 2 (sex) ANOVA for percent body weight gain from day 1 to day 5 of the stress exposure period revealed significant main effects of stress condition, F(1, 40) = 34.10, p < .0001, age, F(1, 40) = 103.34, p < .0001, and sex, F(1, 40) = 10.37, p < .01. Stressed animals gained significantly less weight than non-stressed animals. Younger rats gained significantly more weight than their older counterparts, whereas males gained significantly more weight than females.
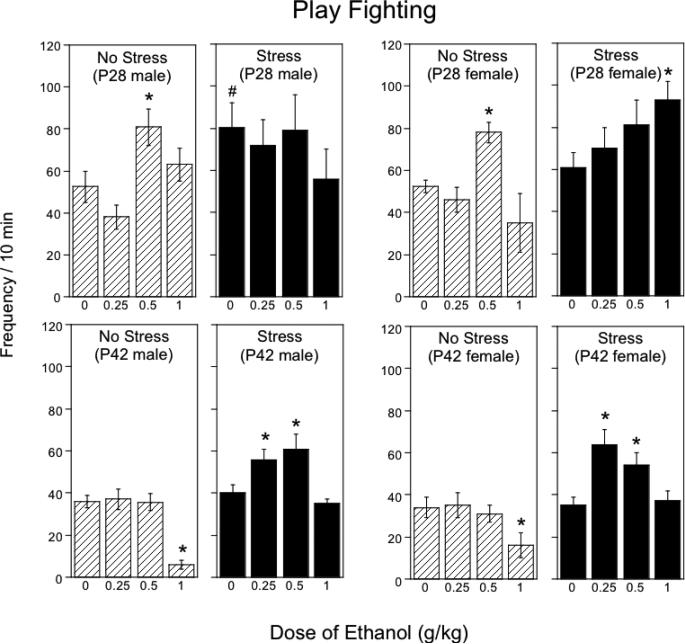
3.2. Play fighting frequency (Fig.1)
Figure 1.
The impact of repeated restraint on ethanol-induced alterations in play fighting in early (P28) and late (P42) adolescent males and females during a 10-min social interaction test. Asterisks (*) indicate significant (p < .05) dose differences from saline-challenged controls within each stress/age/sex condition, pound signs (#) indicate significant indicate significant (p < .05) stress-associated changes in play fighting relative to non-stressed same age and sex animals following acute saline challenge.
The analysis of play fighting in P28 rats revealed a significant stress × sex × ethanol dose interaction, F(3, 80) = 4.39, p < 0.01. In non-stressed adolescent males and females tested at P28, the dose of 0.5 g/kg produced significant increases in play fighting. Restraint stress substantially enhanced baseline levels of play fighting in early adolescent males, with no further ethanol-induced increase evident in these animals. This stress-associated increase in play fighting was not seen in early adolescent females under basal (i.e., saline-challenged) conditions. However, the stressed females were less sensitive to the stimulatory effects of ethanol on play fighting, demonstrating significant ethanol-induced increases in play only following a dose of 1.0 g/kg, in contrast to a dose of 0.5 g/kg, which was effective in non-stressed females.
In P42 adolescents, play fighting differed as a function of stress condition and ethanol dose, F(3, 80) = 5.17, p < .01. Non-stressed late adolescents demonstrated significant suppression of play fighting following the dose of 1.0 g/kg ethanol, with no stimulatory effects evident at lower ethanol doses. Repeatedly stressed late adolescents, however, showed ethanol-induced facilitation of play fighting following acute challenge with 0.25 and 0.5 g/kg ethanol, and were insensitive to ethanol-associated inhibition of play fighting. No sex differences were observed in stress and/or ethanol effects on play fighting in late adolescent animals.
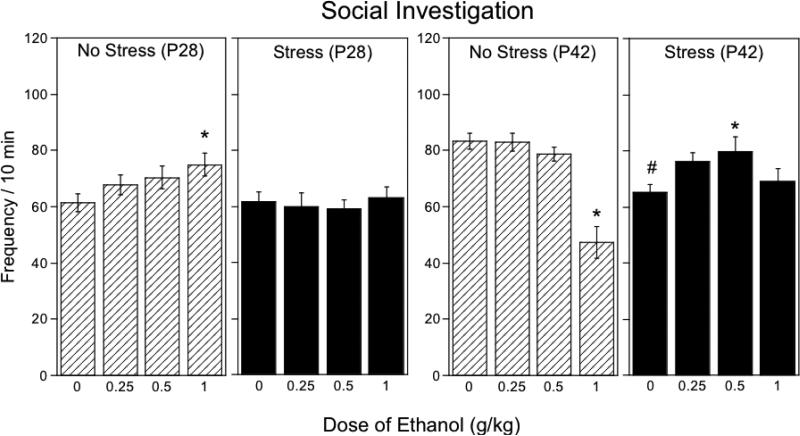
3.3. Social investigation frequency (Fig.2)
Figure 2.
The impact of repeated restraint on ethanol-induced alterations in social investigation in early (P28) and late (P42) adolescent rats during a 10-min social interaction test, with data collapsed across sex. Asterisks (*) indicate significant (p < .05) dose differences from saline-challenged controls within each stress/age condition, pound signs (#) indicate significant (p < .05) stress-associated changes in play fighting relative to non-stressed same age animals following acute saline challenge.
In early adolescent animals, social investigation differed as a function of stress and ethanol dose, F(3, 80) = 2.89, p < .05, with no main effect or interactions involving sex. Repeated restraint produced no effect on baseline levels of social investigation in saline-challenged P28 animals, however the stimulatory effects of the 1.0 g/kg dose seen in non-stressed early adolescents were eliminated by repeated restraint.
In contrast, prior stress exposure of older adolescents substantially reduced baseline levels of social investigation and changed sensitivity to the effects of ethanol [stress × ethanol dose interaction, F(3, 80) = 9.50, p < .0001], with again no effects or interactions involving sex. A stress-induced decrease in social investigation was seen in saline-challenged animals, with this apparent social anxiety reversed by the dose of 0.5 g/kg – a stimulatory effect of ethanol not seen in non-stressed controls.
An ethanol-induced suppression of social investigation was seen at the dose of 1.0 g/kg in non-stressed, but not in stressed, P42 adolescents.
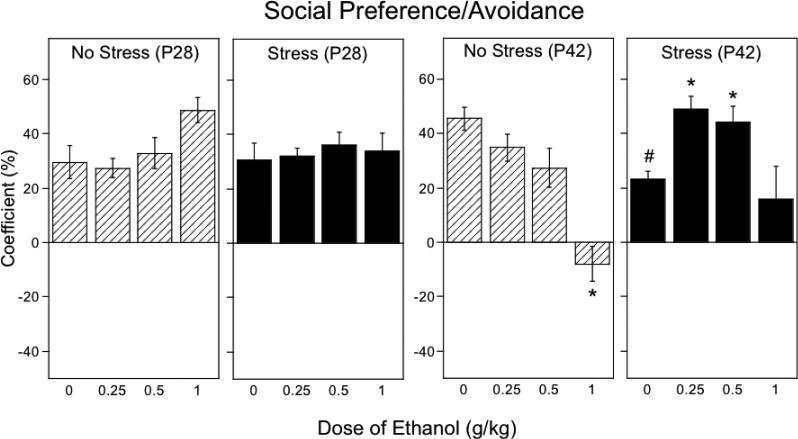
3.4. Social preference/avoidance (Fig.3)
Figure 3.
The impact of repeated restraint on ethanol-induced alterations in social preference/avoidance in early (P28) and late (P42) adolescent rats during a 10-min social interaction test, with data collapsed across sex. Asterisks (*) indicate significant (p < .05) dose differences from saline-challenged controls within each stress/age condition, pound signs (#) indicate significant (p < .05) stress-associated changes in play fighting relative to non-stressed same age animals following acute saline challenge.
In early adolescents, the coefficient was not affected either by ethanol or restraint stress. The ANOVA of the preference/avoidance coefficient in older adolescents revealed a significant stress × ethanol dose interaction, F(3, 80) = 5.57, p < .01. In contrast to younger animals, an anxiogenic effect of repeated restraint emerged in P42 adolescents, with stressed animals challenged with saline showing a reduction of social preference relative to their non-stressed counterparts. This reduction in the social preference coefficient was reversed by ethanol challenge at the doses of 0.25 and 0.5 g/kg. Social preference was transformed into social avoidance in non-stressed P42 animals following 1.0 g/kg ethanol, an effect not evident in stressed animals.
In P42 animals, ethanol dose also interacted with sex, F(3, 80) = 4.41, p < 0.01, with females being less sensitive to ethanol-induced social avoidance than males. When collapsed across stress condition, social avoidance was evident in P42 males following the dose of 1.0 g/kg, whereas in females social avoidance did not emerge at the highest ethanol dose (see Table 2).
Table 2.
Sex-related differences in sensitivity to the social consequences of ethanol among late adolescent (P42) rats, with data collapsed across pre-test stress condition (n=12 per group)
| Ethanol Dose (g/kg) | Social Preference/Avoidance | Crossovers | ||
|---|---|---|---|---|
| male | female | male | female | |
| 0 | 32.3 ± 5.6 | 35.9 ± 4.6 | 38.4 ± 2.1 | 40.6 ± 1.8 |
| 0.25 | 42.6 ± 5.6 | 41.2 ± 5.0 | 39.7 ± 3.0 | 39.8 ± 2.8 |
| 0.5 | 27.2 ± 6.7 | 44.4 ± 6.2 | 33.8 ± 1.9 | 38.5 ± 2.9 |
| 1.0 | −16.2 ± 10.9* | 24.3 ± 7.6 | 16.8 ± 2.6* | 31.4 ± 3.6 |
Asterisks indicate significant differences from corresponding saline controls within each sex.
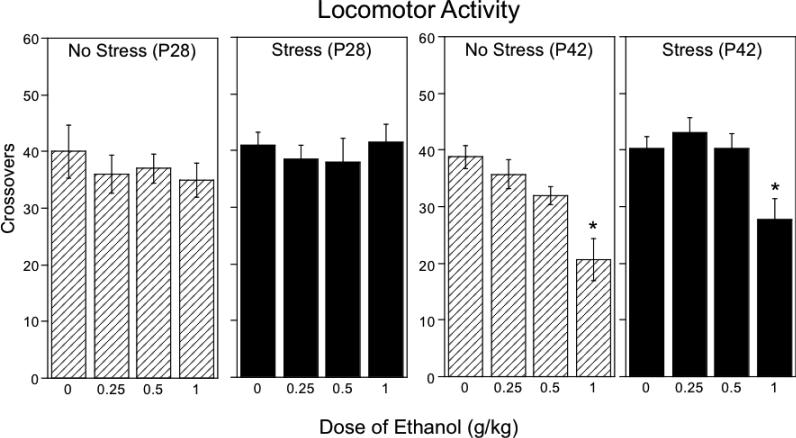
3.5. Locomotor activity under social circumstances (Fig.4)
Figure 4.
The impact of repeated restraint on ethanol-induced alterations in overall locomotor activit in early (P28) and late (P42) adolescent rats during a 10-min social interaction test, with data collapsed across sex. Asterisks (*) indicate significant (p < .05) dose differences from saline-challenged controls within each stress/age condition.
Repeated restraint and acute ethanol challenge had no effect on overall locomotor activity under social circumstances in early adolescents. The ANOVA of total number of crossovers (an index of locomotor activity within the social context) in P42 animals revealed significant main effect of ethanol dose, F(3,80) = 17.17, p < 0.0001, and no effects or interactions involving stress, with a decrease in overall locomotor activity evident at 1.0 g/kg ethanol. This effect, however was tempered by a significant sex × ethanol dose interaction, F(3, 80) = 3.24, p <0.05. When collapsed across stress condition, late adolescent females were less sensitive to ethanol challenge than their male counterparts, with these sex differences evident at the 1.0 g/kg dose of ethanol (see Table 2).
3.6. Blood ethanol concentration (Table 3)
Table 3.
Blood Ethanol Concentration in non-stressed and stressed P28 and P42 rats, with data collapsed across sex (n=12 per group)
| Ethanol Dose (g/kg) | BEC (mg/dl) P28 | BEC (mg/dl) P42 | ||
|---|---|---|---|---|
| No Stress | Stress | No Stress | Stress | |
| 0.25 | 15.8 ± 0.7 | 13.8 ± 0.9 | 16.8 ± 0.6 | 17.3 ± 1.5 |
| 0.5 | 20.3 ± 0.8 | 25.6 ± 0.9 | 23.8 ± 1.7 | 23.6 ± 2.0 |
| 1.0 | 72.9 ± 4.6 | 75.3 ± 4.3 | 84.7 ± 4.4 | 88.3 ± 5.6 |
BECs increased in a dose-dependent fashion in early and late adolescents, [main effects of ethanol dose, F(2, 60) = 602.73, p < 0.0001 and F(2, 60) = 439.01, p < 0.0001, respectively), but did not differ as a function of sex or stress at either age (see Table 2).
4. Discussion
Findings of the present study demonstrate that responsiveness to restraint stress in terms of both stress-induced behavioral alterations and stress-associated changes in sensitivity to the social consequences of acute ethanol challenge differs drastically among early adolescent and late adolescent rats. However, physiological consequences of repeated restraint are similar among early and late adolescents. Restraint stress significantly suppressed body weight gain at both ages, suggesting that the procedure was equally stressful for younger and older animals.
4.1. Stress-associated behavioral alterations
Repeated restraint increased anxiety-like behavior in a social context when indexed both via decreases in social investigation and social preference in older adolescents, but not in their younger counterparts. In our previous studies, repeated restraint stress that occurred either on P31-P35 or on P65-P70 also induced reliable anxiety-like behavioral alterations with these same measures (Varlinskaya et al., 2010), although adult males did not show the significant stress-induced reduction in social preference (Doremus-Fitzwater et al., 2009). Consequently, two measures of social behavior of mid adolescents and adults, namely social investigation and social preference, are exceptionally sensitive to the anxiogenic effects of repeated restraint stress. Therefore, it was not surprising that, in the present study, late adolescent males and females demonstrated significant decreases in social investigation and social preference following repeated restraint.
Unexpectedly, prior repeated restraint that occurred during the juvenile period did not alter social investigation and social preference in animals tested as early adolescents on P28. Instead, early adolescent males responded to the prior stress exposure with an enhancement of an adolescent-characteristic form of social behavior, namely, play fighting. One of the possible explanations for these drastic age-related differences in the consequences of repeated restraint is that early adolescent animals tested at P28 do not respond to anxiety-provoking manipulations in a way their older counterparts do. It is unlikely, however, given that early adolescent rats, in a manner similar to their more mature counterparts, respond to a novel, anxiety-provoking test situation by transformation of social preference into social avoidance (Varlinskaya & Spear, 2006, 2008a).
An alternative possibility is that the 90-min periods of restraint were perceived by juvenile males as significant social deprivation. Indeed, repeated social deprivation (90 min/day, 5 days) was found to produce substantial increases in play fighting in early-mid adolescent males, but not their female counterparts (Doremus-Fitzwater et al., 2009), with the most pronounced activating effects on play fighting evident following social deprivation between P23 and P28, (Varlinskaya & Spear, 2008). Similarly, sex differences in play fighting have been reported for socially deprived juvenile rats, with males engaging in more play fighting than females (see Vanderschuren et al., 1997 for references and review), suggesting that males are more sensitive than females to the activating effects of social deprivation on play fighting during this developmental period. Interestingly, stressful events that occur even earlier in ontogeny can also enhance play fighting in young adolescents. For instance, maternal separation (3 hr/day for 14 days) resulted in enhanced play fighting, when males were tested at P35 (Veenema & Neumann, 2009). Therefore, adverse early experiences that include social deprivation are likely to enhance the adolescent-typical social behavior of play fighting in male rats.
In contrast to their younger counterparts, late adolescents responded to stress exposure by significant decreases in social investigation and social preference. Similar to our previous findings in mid adolescents and adults (e.g., Doremus-Fitzwater et al., 2009; Varlinskaya et al., 2010), the anxiogenic consequences of repeated restraint during late adolescence were specific to these two behavioral measures, with baseline levels (i.e., levels of saline-injected animals) of play fighting and locomotor activity being unaffected by repeated restraint. Taken together with our earlier research (Doremus-Fitzwater et al., 2009; Varlinskaya et al., 2010), these findings suggest that stress-induced alterations in social preference and/or social investigation are age-dependent and cannot be observed if repeated exposure to the stressor occurred prior to P28.
In the rat, the developmental period between weaning and postnatal day 28 corresponds to the pre-pubertal or juvenile stage of development in both males and females (Schneider, 2013; Vetter-O'Hagen & Spear, 2012). Some researchers suggest that pre-pubertal animals differ dramatically in their responsiveness to stress relative to post-pubertal, adult rats (Koenig et al., 2012; McCormick and Mathews, 2007; Romeo, 2010; Romeo et al., 2004a,b). Pre-pubertal stress has been shown to have long-lasting consequences. For instance, alterations in stress responsiveness in adulthood were evident following even a brief, acute stress exposure on P28 (Avital & Richter-Levin, 2005). Furthermore, pre-pubertal stress enhanced anxiety-like behavior and substantially reduced exploratory behavior in adulthood (Jakobson-Pick & Richter-Levin, 2010; Tsoory et al., 2007). However, when these young animals were tested immediately after exposure to stressors, they demonstrated increases in exploratory behavior (Horovitz et al., 2012) and decreases in anxiety (Javcobson-Pick & Richter-Levin, 2012), findings that are reminiscent of the results of the present study. Therefore, it is possible that the observed age differences in the social consequences of exposure to restraint are related, to some extent, to pubertal maturation, with the social behavioral manifestations differing dramatically in pre-pubertal (P28) and peri-pubertal (P42) animals.
4.2. Stress-associated alterations in responsiveness to acute ethanol challenge
In accord with our earlier findings (Varlinskaya and Spear, 2004, 2006), considerable age-related differences in sensitivity to the social consequences of acute ethanol challenge were observed among non-stressed adolescents, with only P28 animals, but not their older counterparts tested at P42 demonstrating ethanol-induced facilitation of play fighting and social investigation. In contrast, no inhibitory effects of ethanol on social behavior and locomotor activity were evident in young non-stressed adolescents, whereas older non-stressed adolescents demonstrated significant social and locomotor inhibition following the highest ethanol dose. It is possible that these inhibitory effects of ethanol evident at the 1.0 g/kg dose in late adolescents may have reflected ethanol-induced sedation, with suppressed locomotor activity contributing to social inhibition. This possibility seems less likely, however, given that this suppressant effect in late adolescents on the total number of crossovers was seen in both non-stressed and stressed animals, whereas ethanol-induced social suppression was only evident in the non-stressed animals.
Alterations in sensitivity to the social consequences of acute ethanol following repeated restraint in late adolescents were not limited to the apparent stress-associated sobering effects. Similarly to adults (Varlinskaya et al., 2010), animals stressed in late adolescence expressed ethanol-induced facilitation of play fighting that is normally evident in early and mid adolescents, but not older animals, after administration of low doses of ethanol (Varlinskaya and Spear, 2002, 2004, 2006). These stress effects were evident in both males and females. Ethanol-induced increases in play fighting were not associated with any increases in locomotor activity when indexed via total number of crossovers in the social test context, suggesting that these activating effects of ethanol reflect ethanol-induced social facilitation rather than general activation.
Stressed females tested at P28 still demonstrated ethanol-associated facilitation of play fighting, although they required a higher ethanol dose relative to their non-stressed counterparts for this effect to emerge (1.0 g/kg versus 0.5 g/kg). In contrast, repeated restraint stress eliminated sensitivity to the stimulatory effects of ethanol in P28 adolescent males, with no ethanol-induced facilitation of play fighting or social investigation evident at any dose. The lack of ethanol-induced facilitation of play fighting in stressed P28 males is likely related, at least in part, to the dramatic stress-associated increase in baseline levels of this adolescent-characteristic form of social interactions from which it might be difficult to see further stimulatory effects.
Our previous research focusing on neural mechanisms of the ethanol-induced social facilitation typically evident in adolescence has shown this social facilitation to be associated, at least in part, with ethanol-induced activation of the endogenous mu opioid receptor (MOR) system (Varlinskaya & Spear, 2009). This finding was not surprising, given that the MOR system is implicated in modulation of play behavior, with selective agonists increasing play fighting in young adolescent animals and antagonists having an opposite effect (see Trezza et al., 2010 for references and review). Taken together, these findings suggest that under normal, non-stressful circumstances, ethanol may produce more pronounced activation of the endogenous MOR system in younger than in older adolescents and in adults, with this activation inducing facilitation of play fighting during early and mid adolescence, but not in late adolescence or adulthood. Repeated restraint, however, makes older animals extremely sensitive to the socially facilitating effects of ethanol, and these effects of ethanol in stressed animals may be MOR-related as well. Indeed, adult rats were found to be insensitive to a selective MOR agonist DAMGO under normal, non-stressful circumstances, whereas stressed adults showed significant increases in play fighting following DAMGO administration (Varlinskaya & Spear, 2008b). In contrast, previously stressed young adolescents became insensitive to the socially activating effects of this selective MOR agonist, suggesting that exposure to stressors produces alterations within the MOR system, with these alterations being age-dependent (Varlinskaya & Spear, 2008b).
Although the endogenous MOR system plays a substantial role in ethanol-induced social facilitation during adolescence (Trezza et al., 2009; Varlinskaya & Spear, 2009), some experimental evidence implicates other neural systems in the modulation of play fighting. For instance, indirect cannabinoid agonists (Trezza & Vanderschuren, 2008a,b) have also been shown to facilitate social behavior during adolescence, with CB1 receptor antagonists altering ethanol-induced increases in play behavior in adolescents (Trezza et al., 2009), and the endogenous cannabinoid system implicated in ethanol intake and reinforcement (Vengeliene et al., 2008) and stress responsiveness (Hill & Tasker, 2012; Riebe & Wotjak, 2011). Play fighting in adolescent rats is also under inhibitory control of the NMDA system, with NMDA antagonists facilitating play fighting at low doses, but suppressing social behavior at higher doses (Siviy et al., 1995) – biphasic effects on play fighting similar to those induced by ethanol (Varlinskaya & Spear, 2002, 2006). Our recent study has also supported the hypothesis that the increases in social interactions observed in adolescents following acute ethanol may be driven in part by NMDA receptor antagonism—particularly of the NR2B subunit—given that a selective NR2B antagonist, ifenprodil, facilitated play fighting in a manner similar to that produced by low doses of ethanol (Morales et al., 2013). Therefore, a number of neural systems, including endogenous cannabinoid, NMDA, and opioid systems may contribute to stress-induced alterations in play fighting observed in young adolescent males and developmental alterations in sensitivity to the effects of ethanol on social behavior.
Stress-induced social anxiety-like behavioral alterations seen in older adolescents were reversed by ethanol, suggesting an enhanced sensitivity to the socially anxiolytic effects of ethanol in these animals. This stress-associated enhancement of sensitivity to ethanol anxiolysis may be related in part to stress-induced alterations in the GABAA receptor system that has been shown to contribute to a number of ethanol effects (Enoch, 2008; Kumar et al., 2009; Lobo & Harris, 2008), including its anxiolytic properties (Eckardt et al., 1998). GABAA receptors with different subunit composition appear to differentially contribute to various ethanol effects, with α1 subunits playing a role in ethanol-induced sedation and motor impairment (Werner et al., 2006), and α2/α3 subunits implicated in anxiolytic effects of ethanol (Morris et al., 2006). Exposure to stressors has been shown to increase expression of α2 subunits in brain regions associated with anxiety (Jacobson-Pick et al., 2012), and these stress-associated changes in GABAA subunit expression may play a role in the enhanced sensitivity to the anxiolytic effects of ethanol observed in late adolescents. In contrast, younger adolescents demonstrated a decrease in expression of α2 subunits in the amygdala after the same stressors (Jacobson-Pick & Richter-Levin, 2012). These age-dependent alterations in expression of α2 subunits may contribute to the drastic differences in stress-associated social anxiety-like behavioral alterations and sensitivity to the anxiolytic effects of ethanol that are seen between early and late adolescent rats.
The results of the present study clearly demonstrate that the immediate consequences of exposure to stressors in terms of behavioral alterations and changes in ethanol sensitivity differ dramatically in pre-pubertal rats relative to their more mature adolescent counterparts, with older adolescents demonstrating stress-induced decreases in social investigation and social preference as well as enhancement of sensitivity to ethanol anxiolysis. Neural mechanisms of these age differences in stress responsiveness remain to be investigated.
Highlights.
> Early and late adolescent rats responded differently to repeated restraint stress.
> Repeated restraint decreased social investigation and preference in late adolescent.
> In contrast, the stressor enhanced social play in early adolescents.
> Stress enhanced ethanol anxiolysis and social facilitation in late adolescents.
> Restraint made early adolescents insensitive to ethanol-induced social facilitation.
Acknowledgements
Supported by grants R01 AA017355, U01 AA019972 to Linda Spear and AA012453 to Elena Varlinskaya.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlstrom SK, Osterberg EL. International perspectives on adolescent and young adult drinking. Alcohol Res Health 2005. 2005;28:258–268. [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34(12):2106–15. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol. 2005;8(2):163–73. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res. 2011;35(8):1392–403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmer SG, Swanson MR. An Evaluation of ten pairwise multiple comparison procedures by Monte Carlo methods. J Amer Statist Assoc. 1973;68:66–74. [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29(9):1641–53. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29(10):1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97(3-4):484–94. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72(1):114–23. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draski LJ, Bice PJ, Deitrich RA. Developmental alterations of ethanol sensitivity in selectively bred high and low alcohol sensitive rats. Pharmacol Biochem Behav. 2001;70(2-3):387–96. doi: 10.1016/s0091-3057(01)00621-9. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22(5):998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90(1):95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Horovitz O, Tsoory MM, Hall J, Jacobson-Pick S, Richter-Levin G. Post-weaning to pre pubertal (‘juvenile’) stress: a model of induced predisposition to stress-related disorders. Neuroendocrinology. 2012;95(1):56–64. doi: 10.1159/000331393. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S, Richter-Levin G. Short- and long-term effects of juvenile stressor exposure on the expression of GABAA receptor subunits in rats. Stress. 2012;15(4):416–24. doi: 10.3109/10253890.2011.634036. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S, Richter-Levin G. Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats. Behav Brain Res. 2010;214(2):268–76. doi: 10.1016/j.bbr.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S, Audet MC, McQuaid RJ, Kalvapalle R, Anisman H. Stressor exposure of male and female juvenile mice influences later responses to stressors: modulation of GABAA receptor subunit mRNA expression. Neuroscience. 2012;215:114–26. doi: 10.1016/j.neuroscience.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2012. Institute for Social Research, The University of Michigan; Ann Arbor: 2013. [Google Scholar]
- Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol. 1963;166:408–18. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JI, Walker CD, Romeo RD, Lupien SJ. Effects of stress across the lifespan. Stress. 2011;14(5):475–80. doi: 10.3109/10253890.2011.604879. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, et al. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 2009;205(4):529–64. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology (Berl) 1979;64(1):61–5. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Lobo IA, Harris RA. GABA(A) receptors and alcohol. Pharmacol Biochem Behav. 2008;90(1):90–4. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86(2):220–33. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Low doses of the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, induces social facilitation in adolescent male rats. Behav Brain Res. 2013;250C:18–22. doi: 10.1016/j.bbr.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris HV, Dawson GR, Reynolds DS, Atack JR, Stephens DN. Both alpha2 and alpha3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. Eur J Neurosci. 2006;23(9):2495–504. doi: 10.1111/j.1460-9568.2006.04775.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol Clin Exp Res. 1998;22(7):1485–92. [PubMed] [Google Scholar]
- Odell WD. Sexual maturation in the rat. In: Grumbach MM, Sizonenko PC, Aubert ML, editors. Control of the onset of puberty. Williams and Wilkins; Baltimore: 1990. pp. 183–210. [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus Norvegicus. Aggressive Behavior. 1987;13(4):227–42. [Google Scholar]
- Petersen AC, Silbereisen RK, Sorensen S. Adolescent development: A global perspective. In: Hurrelmann K, Hamilton SF, editors. Social Problems and Social Contexts in Adolescence. Aldine de Gruyter; New York: 1996. pp. 3–37. [Google Scholar]
- Ramirez RL, Spear LP. Ontogeny of ethanol-induced motor impairment following acute ethanol: assessment via the negative geotaxis reflex in adolescent and adult rats. Pharmacol Biochem Behav. 2010;95(2):242–8. doi: 10.1016/j.pbb.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe CJ, Wotjak CT. Endocannabinoids and stress. Stress. 2011;14(4):384–97. doi: 10.3109/10253890.2011.586753. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52(3):244–53. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004a;79(3):125–32. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004b;80(6):387–93. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Schneider M. Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 2013 doi: 10.1007/s00441-013-1581-2. Epub 2013/02/23. doi: 10.1007/s00441-013-1581-2. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, et al. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34(12):2061–9. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22(3):670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Line BS, Darcy EA. Effects of MK-801 on rough-and-tumble play in juvenile rats. Physiol Behav. 1995;57(5):843–7. doi: 10.1016/0031-9384(94)00361-8. [DOI] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. Norton; New York: 2010. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010;52(3):236–43. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: differential behavioral mechanisms. Eur Neuropsychopharmacol. 2008a;18(7):519–30. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology (Berl) 2008b;197(2):217–27. doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. Prosocial effects of nicotine and ethanol in adolescent rats through partially dissociable neurobehavioral mechanisms. Neuropsychopharmacol. 2009;34(12):2560–73. doi: 10.1038/npp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31(10):463–9. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol. 2007;17(4):245–56. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21(3):309–26. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26(10):1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann NY Acad Sci. 2004;1021:459–61. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48(2):146–61. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008a;188(2):398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social Facilitation induced by pharmacological activation of mu opioid receptors: Impact of age, sex and stress.. Poster presented at the annual meeting of the Society for Neuroscience; Washington, DC. 2008b, November. [Google Scholar]
- Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res. 2009;33(6):991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacol Biochem Behav. 2012;100(3):440–50. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96(2):228–35. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol Behav. 1999;67(4):475–82. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol Clin Exp Res. 2001;25(3):377–85. [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology. 2009;34(3):463–7. doi: 10.1016/j.psyneuen.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154(2):299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31(7):1159–68. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44(6):547–54. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O'Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev Psychobiol. 2012;54(5):523–35. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Girotti M, Spencer RL. Restraint-induced fra-2 and c-fos expression in the rat forebrain: relationship to stress duration. Neuroscience. 2007;150(2):478–86. doi: 10.1016/j.neuroscience.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner DF, Blednov YA, Ariwodola OJ, Silberman Y, Logan E, Berry RB, et al. Knockin mice with ethanol-insensitive alpha1-containing gamma-aminobutyric acid type A receptors display selective alterations in behavioral responses to ethanol. J Pharmacol Exp Ther. 2006;319(1):219–27. doi: 10.1124/jpet.106.106161. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, et al. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73(3):673–7. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Willey AR, Varlinskaya EI, Spear LP. Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav Brain Res. 2009;202(1):122–9. doi: 10.1016/j.bbr.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30(2):141–50. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]