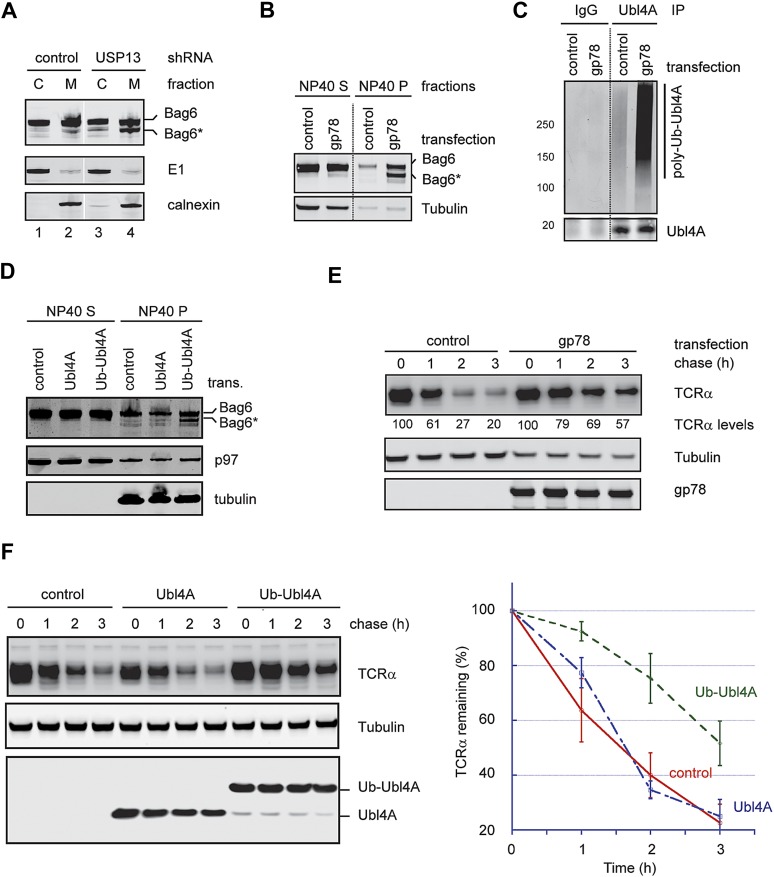
Figure 7. Hyper-ubiquitination of Ubl4A is associated with Bag6 clipping and ERAD inhibition.
(A) Membrane-associated Bag6 is preferentially cleaved in USP13 knockdown cells. C, cytosol fraction; M, membrane fraction. (B and C) Overexpression of gp78 causes cleavage of Bag6. (B) A fraction of the cells transfected as indicated were extracted sequentially with NP40- and SDS-containing buffers. The corresponding extracts were analyzed by immunoblotting. (C) Protein extracts from HEK293 cells transfected with control or gp78-expressing plasmid were subject to immunoprecipitation with Ubl4A antibodies or with IgG as a negative control. (D) Overexpression of a Ub-Ubl4A fusion protein induces Bag6 cleavage. As in B, except that cells expressing the indicated proteins were analyzed. (E) Overexpression of wild-type gp78 inhibits TCRα degradation. The number indicates the relative levels of TCRα averaged from two independent experiments. (F) Overexpression of Ub-Ubl4A inhibits TCRα degradation. The graph shows the quantification results from three independent experiments. Error bars, SD (n = 3).