Figure 1. Descriptions of prototypical CARs and genetically modified T cells from which they are expressed.
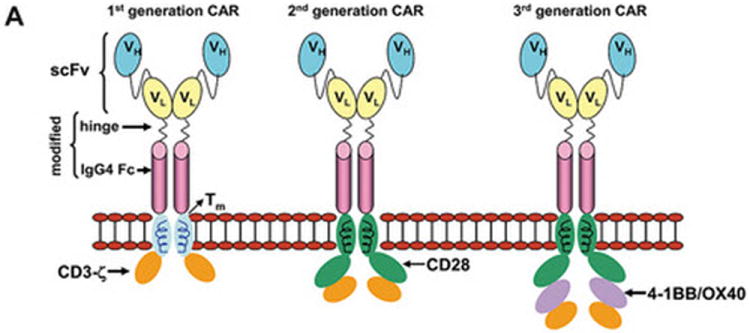
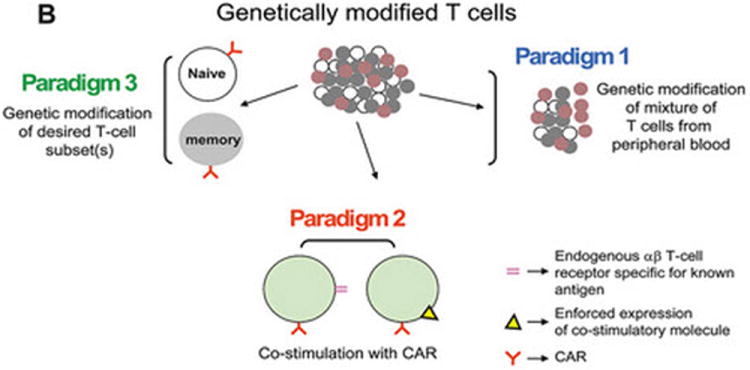
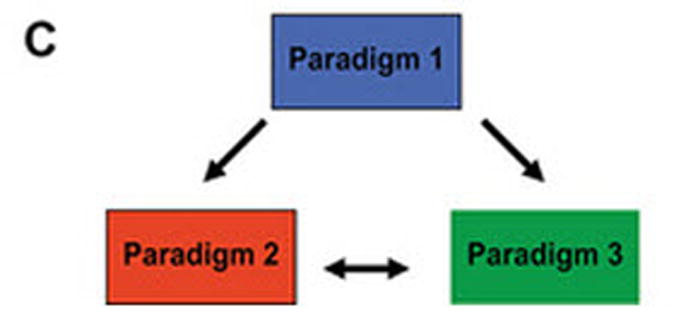
(A) Dimerized CARs demonstrating the extracellular scFv (VH linked to VL) via a linker) region, linked to a flexible hinge and Fc region (for example, from IgG4) fused to intracellular signaling motifs via a transmembrane domain. The 1st generation CAR is shown as activating T cells through an endodomain composed of only CD3-ζ. The 2nd generation CAR activates T cells through chimeric CD3-ζ and CD28. The 3rd generation CAR activates T cells through three signaling motifs, e.g., CD3-ζ with CD28 and CD134 or CD137. The modular structure of the extracellular and intracellular domains can be readily altered to achieve fully competent CAR-dependent signaling. (B) Paralleling the design changes to CAR is an understanding that the type of T cell into which the CAR is expressed can impact the therapeutic potential of adoptive immunotherapy. Paradigm 1 refers to the collection of (naïve, memory, effector) T cells in peripheral blood which can be genetically modified to express just the CAR without further manipulation. Paradigm 2 generates CAR+ T cells that can signal with other desired receptor(s) such as endogenous αβ TCR or introduced co-stimulatory molecule(s). Paradigm 3 expresses the CAR in desired T-cell subsets such as naïve or central memory. (C) The three proposed paradigms are not mutually exclusive of each other as T cells from peripheral blood can be used as cellular templates for Paradigms 2 and 3. Clinical trials are needed to determine whether the CAR design or the type of T cell from which the CAR is expressed, or both, will result in superior persistence and anti-tumor response.
