Figure 5.
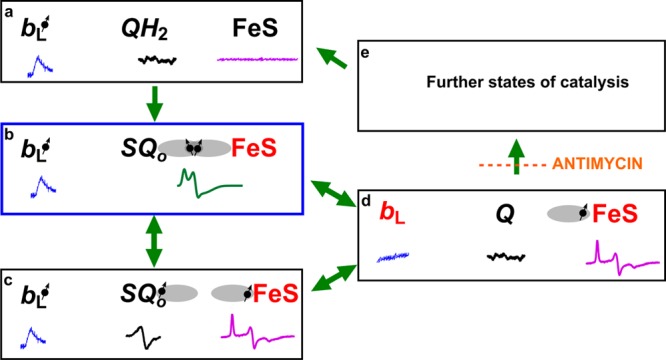
Model of electronic bifurcation of the Qo site accommodating the SQo–FeS coupled system. (a) Bound QH2 is flanked by oxidized heme bL and oxidized FeS. (b) FeS withdraws an electron from QH2, which leads to the formation of the SQo–FeS triplet state. (c) The SQo–FeS distance increases (by movement of the FeS head domain and/or SQo), breaking spin exchange interaction, exposing separate spectra of SQo and reduced FeS. (d) Heme bL is reduced by SQo generating Q. (e) In the noninhibited enzyme, heme bL rapidly transfers an electron across the membrane to heme bH directly or through heme bL in the other monomer44,45 (not shown). The enzyme goes through further states to reach the initial state a. Antimycin prevents oxidation of heme bH, interrupting the transition from state d to state e. Black and red denote the oxidized and reduced cofactor, respectively, while the dot with an arrow indicates the paramagnetic state of the center. Orbitals engaged in spin exchange are shown as gray ovals. Blue, black, magenta, and green spectra are EPR spectra of heme bL, SQo, FeS, and the SQo–FeS triplet state, respectively. Green arrows show transitions between the enzyme states. The blue box denotes the state that was detected as a major fraction of SQ. The scheme does not consider the still unknown proton transfers that may influence transitions between the states.
