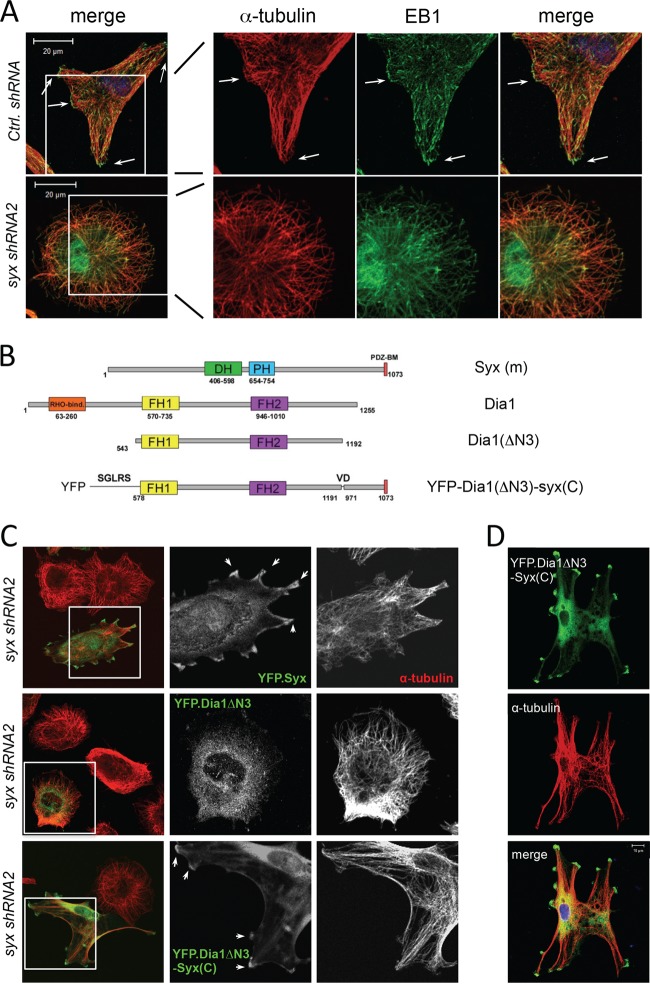
Fig 5.
Syx-targeted constitutively active Dia1 promotes microtubule bundling and multipolar cell morphology. (A) Immunofluorescence staining of the microtubule plus-end capping protein EB1 and α-tubulin in U251 cells expressing control versus syx shRNA. In control cells, large clusters of EB1 puncta are found associated with microtubule bundles at the cell periphery (arrows), whereas Syx-depleted cells lack both large microtubule bundles and the associated EB1 clusters at their tips. (B) Schematic domain structures of murine Syx, Dia1, Dia1ΔN3 (a constitutively active mutant lacking the auto-inhibitory Rho binding domain), and Dia1ΔN3-Syx(C) (conjugation of the last 100 amino acids of Syx to the activated Dia1 mutant). DH, Dbl homology; PH, pleckstrin homology; PDZ-BM, PDZ-binding motif; FH, formin homology. (C) Immunofluorescence staining of Syx-depleted (syx shRNA2) U251 cells expressing pEYFP-Syx, pEYFP-Dia1ΔN3, or pEYFP-Dia1ΔN3-Syx(C). The framed regions are shown separately to highlight the localization of expressed constructs; arrows indicate pEYFP-Syx or pEYFP-Dia1ΔN3-Syx(C) that localized and induced microtubule bundles at cell edges. Fixed cells were stained for YFP and α-tubulin. (D) Immunofluorescence staining of Syx-depleted (syx shRNA1) U251 cells transiently expressing YFP-Dia1ΔN3-Syx(C), which induces microtubule bundling and a multipolar cell morphology similar to that seen with ROCK inhibition in Fig. 4A.