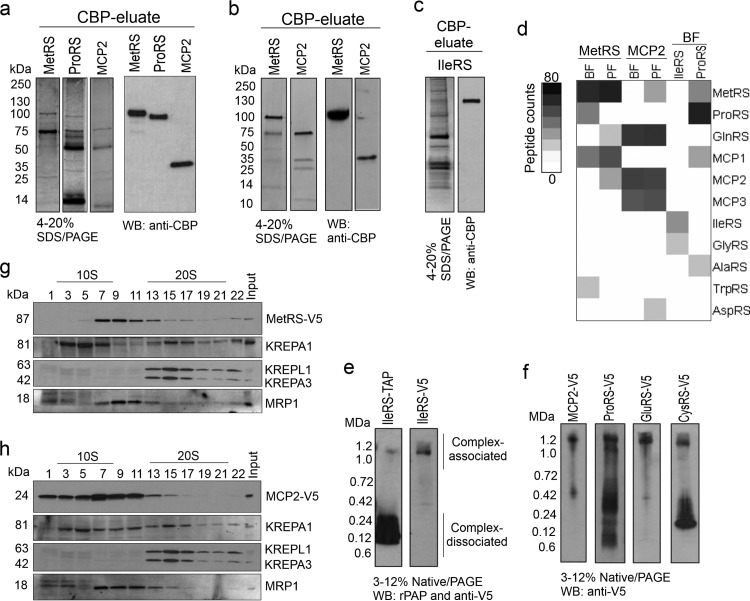
Fig 3.
T. brucei aminoacyl-tRNA synthetase multiprotein complex. (a to c) TAP-tagged purified aaRS examined by SDS-PAGE (4 to 20%) and Western blotting (WB) in which the gels and blots were probed with polyclonal antibodies to anti-CBP. (a) MetRS, ProRS (cytoplasmic; the MTS was removed), and MCP2 from bloodstream forms (BF) (SYPRO Ruby stain). (b) MetRS and MCP2 from procyclic forms (PF) (Imperial stain). (c) IleRS (cytoplasmic; the MTS was removed) from bloodstream forms (silver stain). (d) Intensity map of peptide spectral counts of TAP-tagged proteins that were purified from bloodstream forms and procyclic forms and identified by mass spectrometry. The data summarize the results of two independent TAP tag purifications and mass spectrometry analyses (Table 5). Table S1 in the supplemental material gives a list of all identified proteins. (e) Native PAGE (3 to 12%; Life Technologies) and Western blotting of T. brucei bloodstream forms expressing IleRS-TAP or IleRS-3V5 tags. Protein expression was induced with 100 ng/ml of tetracycline. Protein lysates from 1.0 × 107 parasites per lane were analyzed. The TAP tag was detected with the rPAP reagent (Sigma-Aldrich), and the 3V5 tag was detected with anti-V5 monoclonal antibody. rPAP, rabbit peroxidase antiperoxidase (f) Native PAGE (3 to 12%; Life Technologies) and Western blotting of T. brucei bloodstream forms expressing 3V5-tagged MCP2, ProRS, GluRS, and CysRS. Protein lysates from 1.0 × 107 parasites were analyzed per lane, and the 3V5 tag was detected with anti-V5 monoclonal antibody. Note that GluRS-3V5 and CysRS-3V5 were not identified by tandem affinity purification and mass spectrometry in experiments whose results are shown in Fig. 1d, likely due to weak affinity to the complex or interference of their interaction with tagged proteins or because they were not directly interacting with tagged proteins/complexes. (g) Western blot analysis of glycerol gradient (10 to 30%) fractions of lysate from bloodstream forms expressing 3V5-tagged MetRS probed with anti-V5 monoclonal antibody and then stripped and reprobed with monoclonal antibodies against editosome proteins KREPA1, KREPA3, and KREPL1 (36) and MRP1 (55). (h) Similar to panel g, but with lysate from T. brucei bloodstream forms expressing 3V5-tagged MCP2.