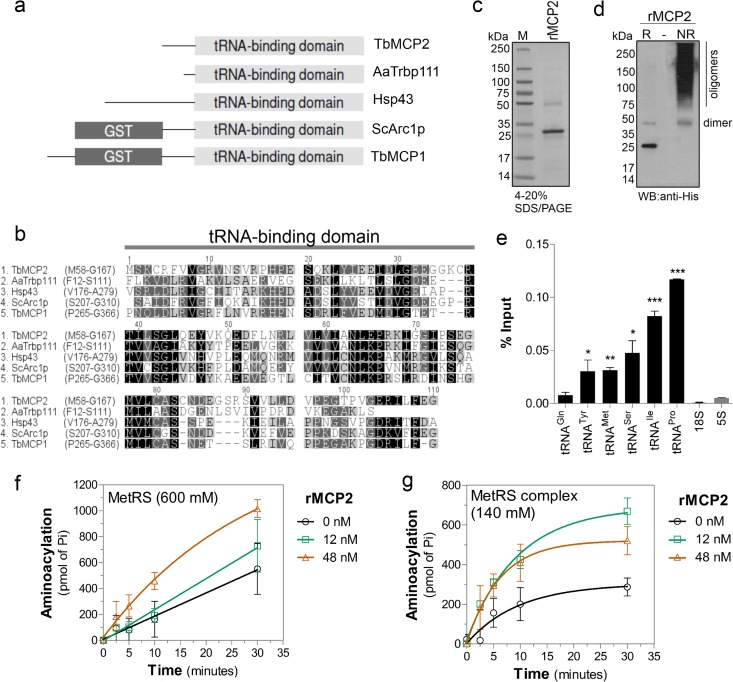
Fig 5.
MCP2 enhances tRNA-aminoacylation by the MARS complex. (a) Diagram of domains conserved in proteins associated with aaRS complexes, including Tb927.7.2400 (T. brucei MCP1 [TbMCP1]) and Tb927.8.5330 (T. brucei MCP2 [TbMCP2]), Saccharomyces cerevisiae Arc1p (ScArc1p; GI: 1620460), Homo sapiens Hsp43 (GI: 85700432), and Aquifex aeolicus Trbp111 (AaTrbp111; GI: 34810888), showing the conserved TRBD and GST domains. (b) Amino acid sequence alignment of the TRBDs of the proteins shown in panel a (amino acid positions are shown). (c) SDS-PAGE (4 to 20%) of rMCP2 purified from E. coli (Imperial stain). Lane M, molecular mass markers. (d) Western blot with anti-His tag antibodies of T. brucei rMCP2 run under reducing conditions (lane R; Laemmli running buffer with 5% β-mercaptoethanol and boiling) or nonreducing conditions (lane NR; Laemmli running buffer without β-mercaptoethanol) in 4 to 20% SDS-polyacrylamide gels. (e) Detection of tRNAs from the binding assay with T. brucei total RNA and rMCP2 analyzed by quantitative RT-PCR. The data show the averages of three independent experiments ± SDs; significance tests were performed by unpaired t test comparing tRNA to 5S rRNA data. ***, P < 0.0001; **, P < 0.005; *, P < 0.05. Aminoacylation kinetics of dissociated MetRS (washed with 600 mM NaCl) (f) or the MetRS complex (washed with 140 mM NaCl [Fig. 4]) (g) upon addition of rMCP2. The averages of three independent experiments ± SDs are shown.