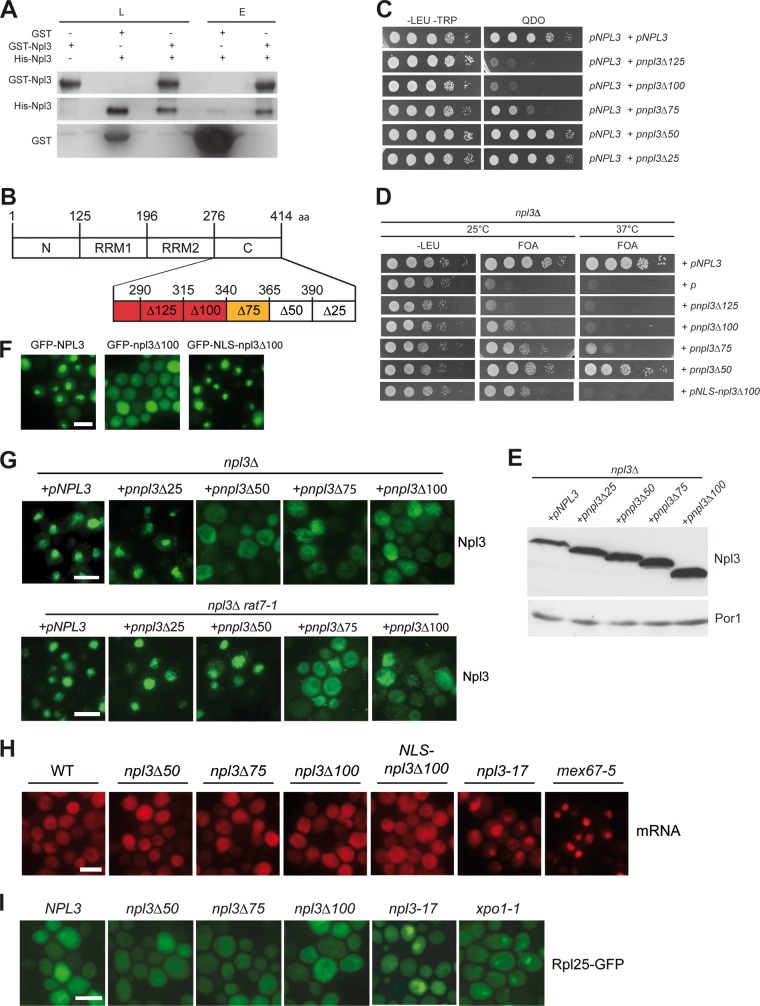
Fig 1.
The dimerization domain of Npl3 is essential for survival but is not crucial for mRNA or pre-60S export. (A) In vitro dimerization of Npl3. Co-affinity precipitations of recombinantly expressed GST-Npl3 with His-Npl3 are shown in the presence of bacterial lysate and RNase A in Western blots. L, lysate; E, eluate. (B) Domain organization of the 414-amino-acid (aa) protein Npl3. N, N-terminal domain; RRM, RNA recognition motif; C, C-terminal domain. Domains necessary (red) or supportive (yellow) for dimerization are also indicated. Deletion mutants used in the present study are indicated by the “Δ” symbol. (C) Two-hybrid analyses reveal a minimal dimerization domain reaching from amino acids 276 to 339 and the full dimerization domain to amino acids 276 to 364. Deletions of the C terminus of Npl3 in 25-amino-acid residue steps were created and cells expressing the truncated versions of Npl3 in combination with wild-type NPL3 were tested for growth on quadruple-dropout (QDO) plates. (D) The C-terminal domain of Npl3 is essential for survival. Truncated versions of NPL3 were subcloned and investigated for the complementation of an npl3 knockout strain. Since the deleted C terminus also lacks the Mtr10 import receptor interaction domain, a nuclear localization signal (NLS) was fused to npl3Δ100 (bottom), allowing Mtr10-independent import. Strains were spotted onto −LEU and 5-fluoorotic acid (FOA) plates on which cells were selected that have lost the covering NPL3 gene on a URA3 vector. (E) The expression of the C-terminal truncation mutants of Npl3 is similar. Western blot analyses of the truncated proteins are shown. Por1 served as a loading control. (F) GFP-NLS-npl3Δ100 is localized to the nucleus. Localization of the indicated GFP-tagged Npl3 variants is shown in a wild-type strain. Scale bars (F, G, H, and I), 5 μm. (G) The nuclear import of npl3Δ100 is inhibited. The localization of wild-type Npl3 and the indicated truncated versions of the protein are shown in npl3Δ and the double-mutant npl3Δ rat7-1 upon a temperature shift to 37°C for 1 h. Antibodies against Npl3 were used for immunofluorescence studies. (H) Dimerization-defective mutants have no mRNA export defects. In situ hybridizations with Cy3-labeled oligo(dT) probes were performed in log-phase wild-type cells, the mex67-5 and npl3-17 mRNA export factor mutants and the indicated truncated npl3 mutant strains shifted to 37°C for 30 min. (I) Dimerization-defective mutants have no pre-60S export defects. The localization of Rpl25-GFP was determined in the wild type, the indicated npl3 mutants, and the 60S export receptor mutant xpo1-1 after a shift to 37°C for 30 min.