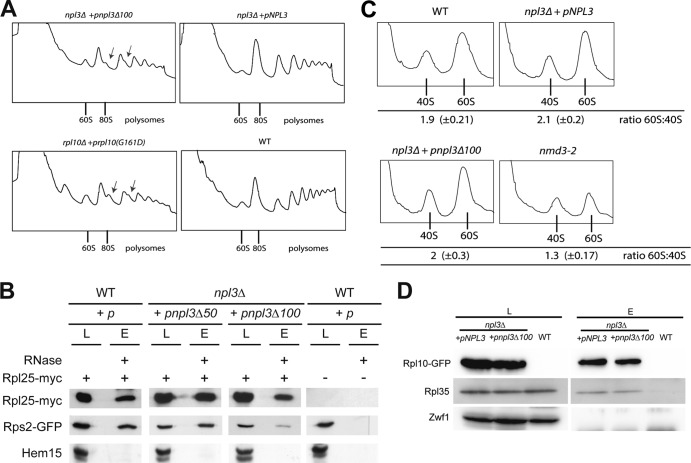
Fig 3.
Npl3 is required for ribosomal subunit joining. (A) The npl3Δ100 mutant is defective in the formation of proper mono- and polysomes. Log-phase wild-type, rpl10(G161D), and npl3Δ100 cells were subjected to sucrose density gradient centrifugation (7 to 47%) upon a shift to 37°C for 30 min. The positions of 60S, the 80S monosomes, and polysomes in the profiles (A254) are indicated. Arrows indicate halfmers. (B) The dimerization of Npl3 is important for the proper interaction of the 40S and 60S ribosomal subunits. Coimmunoprecipitations of Rps2-GFP with Rpl25-myc, indicative for proper 80S formation, were performed in the indicated strains. Western blots including the negative control protein Hem15 are shown. L, lysate; E, eluate. (C) The subunit joining defects in the npl3Δ100 mutant are not due to decreased amounts of ribosomal subunits. Lysates were incubated with EDTA, and the ratio of 60S to 40S ribosomal subunit was determined by sucrose density analyses (7 to 47%) in wild-type, npl3Δ100, and nmd3-2 cells upon a shift to 37°C for 30 min. The ratios of the peak areas of both subunits were calculated from six independent experiments. (D) The incorporation of Rpl10 into the cytoplasmic 60S particle is functional in npl3Δ100 cells. Coimmunoprecipitations of Rpl35 with Rpl10-GFP were compared in wild-type and npl3Δ100 cells in the presence of RNase A. The results are shown in a Western blot. Zwf1 served as a negative control.